The treatment of chronic heart failure, particularly when due to systolic dysfunction, is built around therapies that have been shown to reduce long-term mortality and improve symptoms such as: angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta blockers and aldosterone antagonists. More recent evidence-based management includes device therapy such as Cardiac Resynchronization Therapy (CRT), Implantable Cardiovertor Defibrillator (ICD) or the Ventricular Assist Device (VAD).
In contrast, the goals of the initial management of acute decompensated heart failure (ADHF) are hemodynamic stabilization, correction of the intravascular volume abnormalities, and symptom relief. The main therapy goal for these abnormalities is to lower the ventricular filling (diastolic) pressures by diuretics and vasodilators. The aggressiveness of each treatment depends on the patient’s hemodynamic and volume status.1, 2 Some of the cornerstones of chronic heart failure therapy such as prescribing ACE inhibitors, angiotensin receptors or beta blockers, should either not be used at all, or only used with caution in ADHF, particularly during the period of initial stabilization. Such therapies may be initiated or titrated upward later in a patient’s course (except those patients that already have been on these medications prior to admission).
Management of Acute Heart Failure Symdrome (AHFS) is challenging due to the heterogeneity of the patient population, absence of a universally accepted definition, incomplete understanding of its pathophysiology, and lack of good evidence-based guidelines.
Pulmonary and systemic congestion due to elevated ventricular filling pressures with or without a decrease in cardiac output is a nearly universal finding in AHFS.1, 2 Occasionally, severe pulmonary congestion develops abruptly when precipitated by a rapid increase in systemic blood pressure, particularly in patients with diastolic dysfunction, or ischemia causing sudden rise of left ventricular filling (diastolic) pressure. Pulmonary congestion in this set up is due to fluid redistribution rather than fluid accumulation.
Acute heart failure may be a new case (15-20%) or an exacerbation of chronic disease (80%), with gradually or rapidly worseningheart failure (HF) signs and symptoms requiring urgent therapy orhospitalization. Currently there are only few evidence-based guidelines for acute heart failure and they largely reflect a relative lackof robust data. Clinical practice guidelines for the management ofAHFS have only recently been addressed and many recommendations are from expert consensus. ADHF guidelines have beenpublished by the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) - 2009, the European Society of Cardiology (ESC) - 2005, 2008, and the Heart Failure Society of America (HFSA) - 2010.3-6
For Chronic heart failure (and Acute heart failure)
The definition of heart failure proposed by ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult 7, 8 is as follows:
Heart failure is a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood.
The cardinal manifestations of heart failure are dyspnea and fatigue, which may limit exercise tolerance, and fluid retention, which may lead to pulmonary congestion and peripheral edema.
For acute decompensated heart failure
AHFS is defined as gradual or rapid change in heart failure signs and symptoms resulting in a need for urgent therapy. These symptoms are primarily the result of severe pulmonary congestion due to elevated left ventricular filling pressures (with or without low cardiac output). AHFS can occur in patients with preserved or reduced ejection fraction (EF).1
Table 1 reveals that acute heart failure syndrome (ADHF) patients have significant concomitant diseases. They have a ratio of left ventricular systolic and preserved systolic function of about 50 / 50.
Table 2 reveals that almost all ADHF patients have symptoms or findings of congestion, high left ventricular filling pressure (PCWP = pulmonary capillary wedge pressure) and adequate cardiac output. Most of them have normal or elevated systemic blood pressure. Very few of them actually have blood pressure lower than 90 mmHg.
It is increasingly recognized that many patients with this syndrome have relatively preserved ejection fraction, as many as those patients with depressed systolic function, which indicates that left ventricular ejection fraction is not the only or major factor inducing ADHF. The diastolic (filling pressures) component appears to be thedominant factor and in fact managing ADHF by trying to improve ejection fraction or cardiac output with the available inotropes (dobutamine, milrinone, dopamine) has deleterious effects in most cases (See inotrope section).
Table 1. Demographics and concomitant diseases of acute decompensated heart failure.9-11
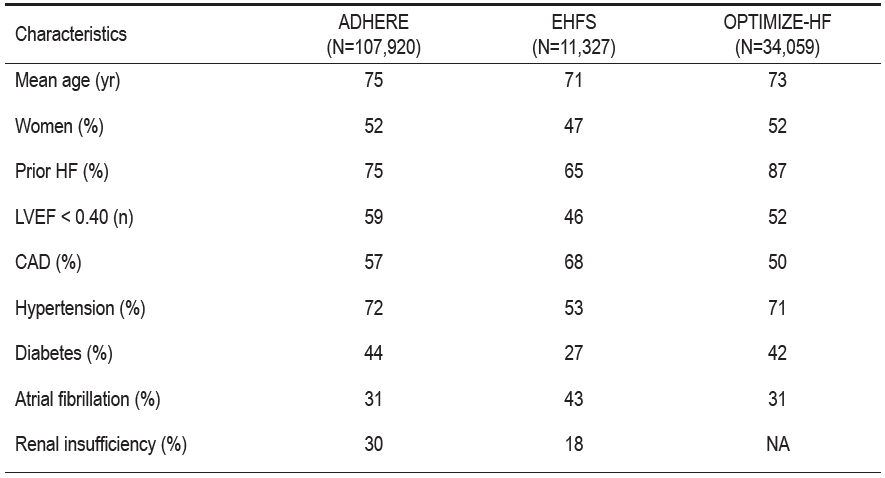
ADHERE = Acute Decompensated Heart Failure National Registry
EHFS = EuroHeart Failure Survey
OPTIMIZE-HF = Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure Registry
CAD = coronary artery disease
HF= heart failure
LVEF = left ventricular ejection fraction
NA = not available.
Table 2: Clinical Presentation of Patients Hospitalized with Heart Failure.12
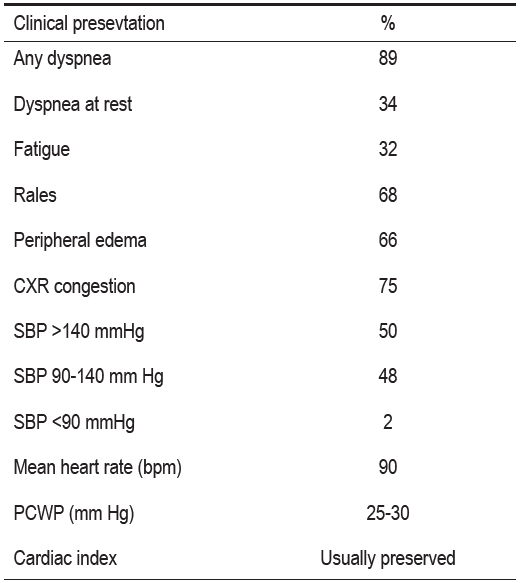
CXR = Chest x-ray
SBP = Systolic blood pressure
PCWP = Pulmonary capillary wedge pressure
Table 3: Diagnostic value of clinical markers of congestion.13, 14
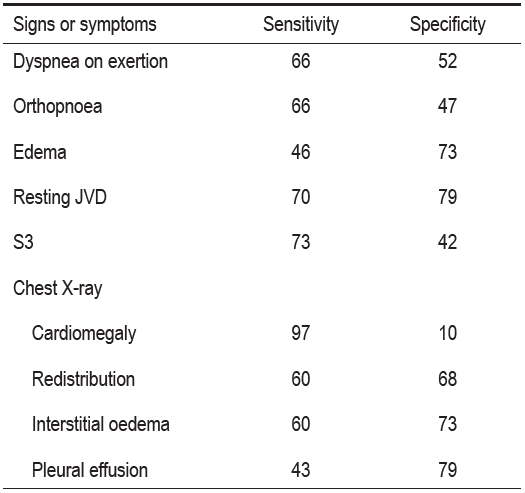
JVD = jugular venous distension
S3 = third heart sound.
All numbers are expressed as percentages.
Note : that Chest x-ray findings are only about two thirds sensitivity and specificity
Table 4: The most useful symptoms and signs15

dominant factor and in fact managing ADHF by trying to improve ejection fraction or cardiac output with the available inotropes (dobutamine, milrinone, dopamine) has deleterious effects in most cases (See inotrope section).
Pulmonary and systemic congestion due to elevated ventricular filling pressures with or without a decrease in cardiac output is a nearly universal finding in ADHF. The degree of fluid retention in each patient is varied. A variety of pathophysiologic mechanisms may play a role in this disorder, many of which remain poorly understood.
Dyspnea on exertion is the most sensitive clinical marker. Paroxysmal nocturnal dyspnea is the most specific, and elevated jugular venous pressure is the best indicator for identifying acute decompensated heart failure, although measurement of jugular venous pressure (JVC) will be inaccurate if it is not performed correctly (Table 4).
It is believed that ventricular abnormalities, systolic or diastolic, activate several neurohormonal systems. The principle neurohormone systems are the reninangiotensin-aldosterone system, and sympathetic nervous system. Others include antidiuretic hormone, arginine vasopressin, vasoconstrictor endothelin, cytokine and vasodilator natriuretic peptide.16, 17 These neurohormone levels are consistently elevated in heart failure patients. Initially these neurohormones will secrete in response to compensatory mechanisms which are initially beneficial. However sustained neurohormone elevation has many deleterious cardiovascular and renal effects. These unfavorable effects include vasoconstriction, tachycardia, myocyte toxicity, cellular structural and molecular change, increasing fibrosis and remoldeling. The compensatory renal sodium and water retention induced by these maladaptations, together with elevated left ventricular filling pressure from various reasons ultimately results in pulmonary congestion and pulmonary edema. Occasionally, severe pulmonary congestion develops abruptly when precipitated by a rapid increase in BP, particularly in patients with diastolic dysfunction or sudden rise of left ventricular diastolic pressure from ischemia. Pulmonary congestion in this set up is due to fluid redistribution rather than fluid accumulation.
To further validate the significant involvement of neurphormones, several pharmacologic interventions have resulted in improving both mortality and morbidity.18-24
Congestion
High left ventricular and right ventricular diastolic pressure resulting in pulmonary and systemic congestion with or without low cardiac output is the main reason for the majority of acute heart failure admissions. An oversimplified explanation of the development of cardiac congestion at the pulmonary or systemic capillary sites is that it is due to the capillary pressure (hydrostatic pressure) exceeding oncotic pressure of that system, originating from the left and right ventricular filling pressures.
Pulmonary congestion may be defined as pulmonary venous hypertension (increased pulmonary capillary wedge pressure (PCWP), often resulting in pulmonary interstitial and alveolar edema. The PCWP should be equal to the left ventricular filling pressure. Systemic congestion manifests clinically by jugular venous distention. Jugular venous pressure should be equal to the right ventricular filling pressure. The degree of peripheral edema and increased body weight can vary.
Occasionally, severe pulmonary congestion develops abruptly when precipitated by a rapid increase in systemic blood pressure (afterload effect), particularly in patients with diastolic dysfunction. Renal impairment, severe neurohormonal or endothelial abnormalities, dietary indiscretion, and certain medications such as non-steroidal anti-inflammatory drugs, glitazones, and first generation calcium-channel blockers, may also contribute to fluid overload.
In the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) trial, PCWP and not cardiac output was a significant predictor of subsequent survival.25
Neck vein examination
Despite several reports and guidelines which indicate the high sensitivity and specificity of jugular venous pressure examination for assessing fluid volume, this method has been significantly underutilized, particularly the semi-quantitative method. The technique is useful for both acute heart failure and chronic heart failure. When combined with other parameters (symptoms, edema, chest rales, tender liver enlargement, etc.), it helps the diagnosis of heart failure. Jugular venous pressure (JVP) examination also assists with both follow up diuresis and estimation of the patient euvolemic (dry) weight. Results from ESCAPE trial showed that if jugular venous pressure is 11 cmH2O, PCWP should be 22 mmHg or higher.25
However the semi-quantitative method of jugular venous pressure examination is technically demanding in many cases. The examiner needs training under supervision from an experienced person. There will be cases where this method of measurement is not feasible.
The semi-quantitative method of jugular venous pressure assessment:
1. Equipment
a. Preferably an adjustable upper body angulation examining bed. (Figure 1)
b. Bright light such as Halogen or LCD with adequately wide beam.(Figure 2)
2. Adjusting upper body height (angulation) for best identification of the highest level of jugular venous pulsation. The patient’s upper body inclining angle varies from patient to patient and in the same patient at different times.
3. Shine the light from different points (directly on the side of the patient’s neck, from the front, from behind, using lighting shadow).
4. Determine the highest point of internal jugular pulsation from both right and left side. The right side may be easier to examine.
5. Measure the height of the internal jugular pulsation by drawing an imaginary parallel line to intersect the straight up line from sternal angle. Measure the height of that straight up line then add 5 cm (approximate distance from sternal angle to right atrial level). The result will be recorded in centimeters of water (cmH2O). (Figure 3)
These flashlights are of various shapes and sizes (usually about one third or half the length and double the body size of a pencil). They require different kinds of battery and are usually inexpensive.

Figure 1: Example of an examination bed

Figure 2: Examples of examination light source
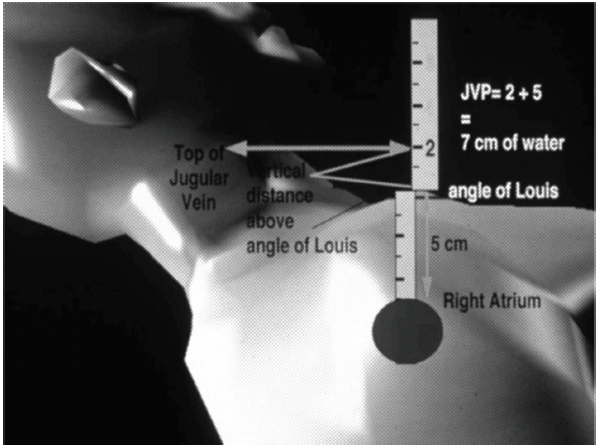
Figure 3: Measurement of jugular venous pressure.
The last 2 decades have seen the successful development of a number of therapies for chronic systolic dysfunction. Angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, β-blockers,aldosterone antagonists, implantable cardioverter defibrillators and cardiac resynchronization therapy have all been shown in large, prospective, randomized controlled trials to reduce morbidity and mortality among patients with stable congestive heart failure and reduced left ventricular ejection fraction.
Unfortunately, the same success has not been seen in the treatment of acute decompensated heart failure. Consequently, therapy for ADHF has not changed significantly over the past 30 years. There have been comparisons made between treatment of decreasing PCWP (= decongestion) and treatment of patients with cardiac output at different levels. It appears that achieving a lower PCWP leads to better immediate long-term mortality results. (Figure 4)
It appears that attempts to diuresis heart failure patients with evidence of fluid retention (after careful assessment of the patient with all appropriate parameters) should not only improve the symptoms (making the patient feel better) but also keep them alive longer.
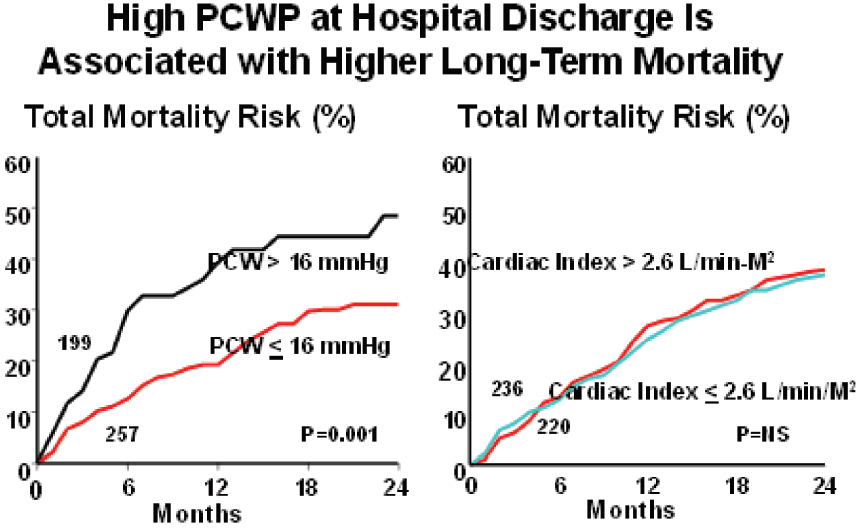
Figure 4: A study by Fonarow26 which demonstrated that if PCWP waslower than 16 mm Hg at discharge, the mortality at 24 months was better than for those whose PCWP was higher than 16 mm Hg. There is no mortality difference between the 2 groups that have cardiac index higher or lower than 2.6 L/min/M2

Figure 5: A study by Lucas C, et al.27 which shows the linear relationship between the degree of congestion and survival rate at 18 months: the less congestion, the better survival rate.
Intravenous therapy with a loop diuretic currently forms the foundation of care for patients with acute decompensated heart failure who have volume overload, but even furosemide use in acute decompensated heart failure is controversial.
Patients with acute decompensated heart failure who present primarily with low cardiac output and renal dysfunction pose a more difficult clinical challenge. Inotropes have traditionally been used in such cases but have consistently failed to show benefit in clinical trials.
o Increased neurohormonal activation
o Electrolyte disturbances and/or arrhythmias
o Worsened renal function
o Metabolic alkalosis, hyperuricemia
Finally it is essential to search for patients’ euvolemic (dry) weight, using the below guidelines:
Reminder
The old concept that heart failure is only due to poor left ventricular systolic function (low ejection) is no longer valid. It is now clear that ADHF occurs in patients with either systolic dysfunction, preserved systolic function or both. The diastolic (filling pressures) component appears to be the dominant factor in ADHF patients. Furthermore, attempts to improve systolic function or increase cardiac output by various inotropes have failed in several trials over the past 20 years. (Table. 5)
Patients with acute decompensated heart failure who present primarily with low cardiac output and renal dysfunction pose a more difficult clinical challenge. Inotropes have traditionally been used in such cases but have consistently failed to show benefit in clinical trials. Data of inotrope utilization frequency continue to be high, suggesting that we are hanging onto the old concept of ejection fraction and cardiac output as the major cause of ADHF whereby it is postulated that the condition can be improved by using inotropes (Table 6).
Table 5: List of inotrope studies over the past 20 years28-44
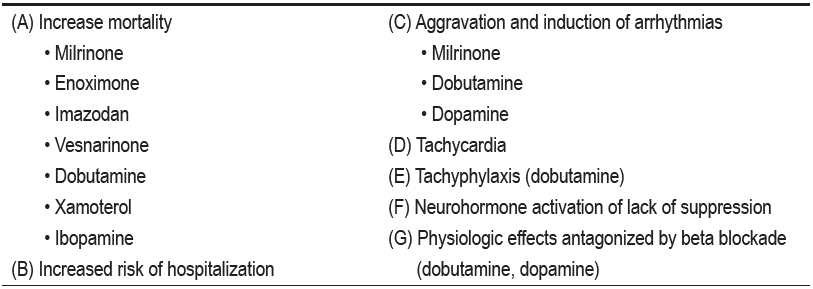
Table 6: Data of inotrope utilization frequency continue to be high
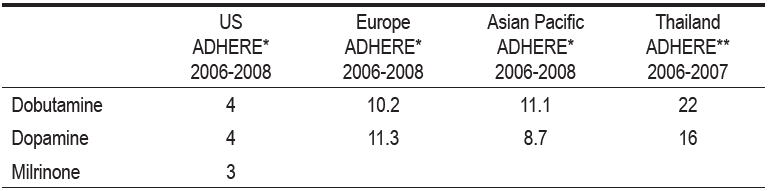
*2nd Interim ADHERE International Report 2006-2008
**Thai ADHERE Registry Report 2006-2007
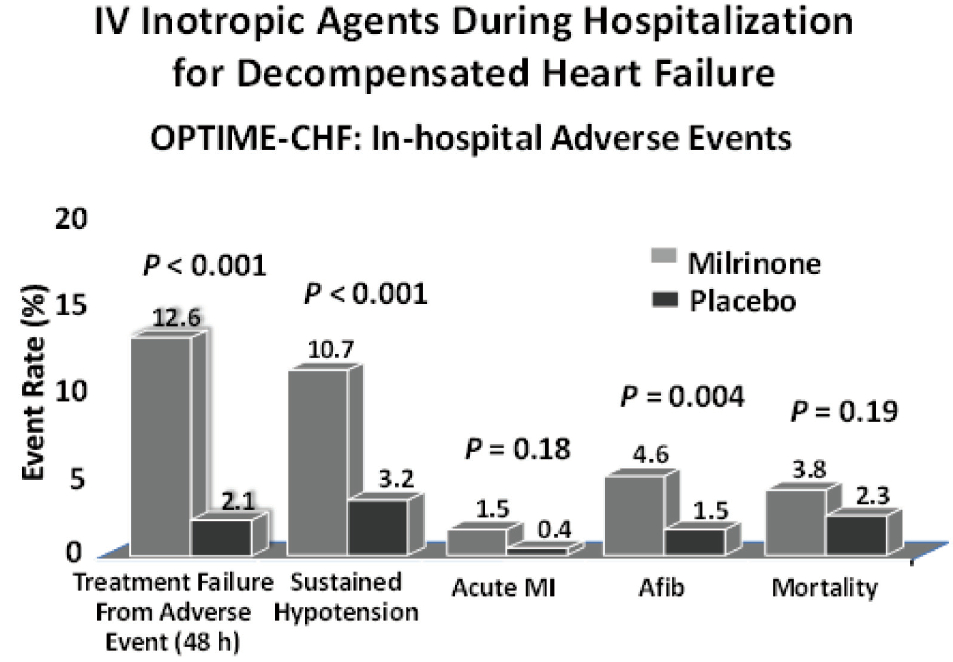
Figure 6: Results of the most recent randomized control trial for milrinone, a phospho diesterest inhibitor inotrope in ADHF. All the end points in this figure showed deleterious effects from milrinone when compared with placebo. These end points included treatment failure from adverse events, sustained hypotension, acute myocardial infarction, atrial fibrillation and mortality; 4 out of 6 had statistic significance.30
1. The routine use of inotropes for heart failure therapy is not indicated in either the short or long term setting.
2. The use of inotropes is potentially appropriate in:
3. Inotropes may be appropriate as a palliative measure in patients with truly end-stage heart failure as part of hospice care.