Vlacyclovir is the L-valyl ester of the antiviral drug acyclovir, first studied in 1983 along with other acyclovir amino acid esters, searching for prodrugs to improve bioavailability and to decrease the toxicity of acyclovir.1 Valacyclovir is chemically stable in aqueous solution and 54% is absorbed after human oral intake, rapidly and extensively converted to acyclovir and amino acid L-valine. Valacyclovir absorption is not altered by administration with food.1-3 Valacyclovir was initially approved in USA in June 1995 and has indications for herpes simplex virus (HSV-1 and HSV-2) and varicella zoster virus infection. The Valacyclovir antiviral activity reflects its in vivo conversion to acyclovir. Acyclovir, which is a nucleoside analogue, is phosphorylated by virally-encoded thymidine kinase and subsequently by cellular enzymes, yielding acyclovir triphosphate, which competitively inhibits viral DNA polymerase so that viral DNA production is terminated. Valacyclovir has more favorable pharmacokinetics requiring less frequent dosing and achieving higher blood plasma levels than acyclovir. Valacyclovir, at a dose of 250 mg four times daily, generates an area under the concentration-time curve (AUC) of acyclovir orally at a dose of 800 mg five times daily.
Valacyclovir, at a dose of 1,000 mg three times daily produces similar acyclovir AUC as intravenous (IV) acyclovir at a dose of 5 mg/kg every eight hours.4,5
Valacyclovir can generally be regarded as an acceptable alternative to oral acyclovir when this drug is indicated and features a more convenient dosing schedule. Generally, Valacyclovir is well tolerated, similar to the experience with acyclovir. The product prescribing information contained warnings and precautions of thrombotic thrombocytopenic purpura/hemolytic uremic syndrome, acute renal failure and central nervous system adverse reactions.2,3,6 Valacyclovir prescriptions in the USA outnumbered acyclovir at least since 2007 by 27% (3,339,913 vs. 2,627,727 prescriptions) and increased by 26.67% in the 10 years to 2017, while acyclovir prescriptions increased by 43.34% in the same period.7 Acyclovir nephrotoxicity is explained by crystal nephropathy and acute interstitial nephritis mechanisms, but acute tubular necrosis can also occur.8,9
A 84-year old female was admitted with a history of unconsciousness. She was found lying on the floor of the common room in her house. She visited this hospital 3 days before with rash on the left arm that developed a day before. Presumptive treatment for Herpes zoster was provided, Valacyclovir 500 mg 2 tablets 3 times a day, methylcobalamin, gabapentin, and levocetirizine. Patient seemed to have slept more frequently but still took meals and medications on schedule. On the admission day, patient took less food than usual for breakfast and lunch, then went for a nap on a sofa-bed as usual. She was later found within half an hour after last being seen, unconscious and unable to be woken up, lying on the floor near the sofa-bed, so her family members decided to call a hospital ambulance.
She had hypertension and idiopathic deep venous thrombosis left leg up to common femoral vein since 2016. Her regular medications from another clinic were amlodipine (5mg/day) nebivolol (2.5mg/day), micronized fenofibrate (200mg/day), warfarin (1mg before bedtime) and piracetam (400g twice a day).
Her consciousness was E3V4M6 at the emergency department. Pupils were 2 mm in diameter, equally reacted to light. Temperature was 37.6 °C, Blood pressure 155/69 mmHg, Pulse 92 beats per minute with regular rhythm, Respiratory rate 18 was breaths per minute with normal respiration pattern. Zoster lesions on the left forearm still contained some vesicles without evidence of on-top bacterial infection. No definite weakness was revealed from her short period of co-operation.
Intravenous (IV) fluid was given on the first day. She was able to take medicines orally and only minimal food. Valacyclovir dosage was continued but dose was reduced to 500mg twice a day due to impaired renal function found. There was retention of urine and a single catheterization yielded 500 ml. Urinalysis showed 1+ proteinuria without white or red blood cell, no crystal seen in urine sediment. Ultrasonography study revealed no renal stone nor obstructing hydronephrosis, urinary bladder full, a small left cortical cyst and no adnexal mass. The renal profile change and fluid intake/output is demonstrated in Table 1.
During the first few days of admission, urine single catheterizations was still needed, with urecholine given (10mg three times a day) until day 4 when she was able to urinate conveniently. Patient consciousness was much improved, able to respond to doctor’s verbal requests, answer questions properly and fair orientation since day 3. However, creatinine level continued to rise on day 4 to 4.22 mg% despite adequate hydration, and good urine output. This raised an awareness of Valacyclovir nephrotoxicity, so the medicine was discontinued. Her clinical appearance remained in good condition, i.e. could take food normally, and urine output was about 2 litres per day. Creatinine level dropped down to 2.3 mg% on day 6 and she was discharged on the subsequent day. Renal function was further improved on follow up as an out-patient five days after discharge, where the creatinine level was only 1.26 mg% and the patient was in a comfortable condition.
Table 1: Renal profile and fluid intake/output during admission 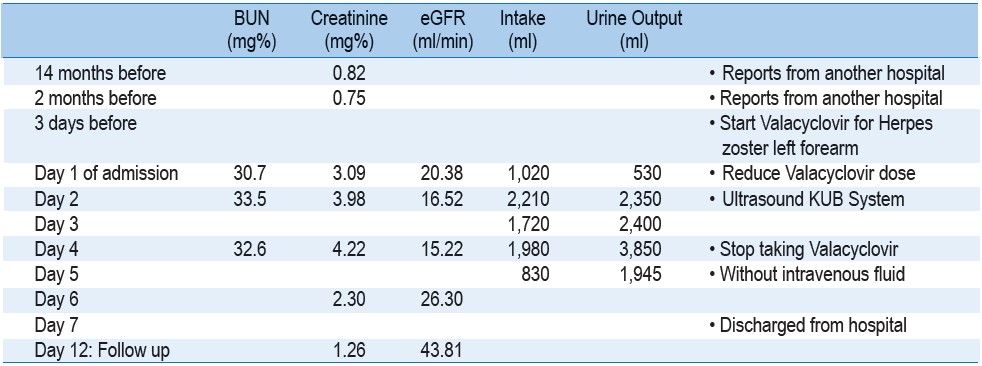
This case report presented a patient with worsened consciousness explained by the drowsiness effect of gabapentin that also reduced her food-fluid intake, further bringing her dehydration to a high dehydration status for her old age.
Her consciousness improved following supportive care and fluid replacement even when she was still taking Valacyclovir at a lower dose, adjusted according to kidney function. In this case awareness of Valacyclovir nephrotoxicity was raised by noticing the creatinine level rising.
Beyond the drug prescription information warnings there is also report of liver injury.10 The kidney injury of Valacyclovir is well known but the risk is not seen to be very high. A population-based study in Ontario, Canada revealed that, from 1997 to 2011, there were 209 events (0.27%) of hospital admission due to acute kidney injury (AKI) in 76,269 patients with older age (71-81, average 76 years) who received new outpatient prescriptions of Valacyclovir or acyclovir.11 Another Japanese study from more than 250,000 reports of adverse drug events were included in the database of the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, a total of 514 kidney-related adverse events were detected, and 344 were cases included AKI. Of the AKI cases, 246 patients (71.5%) were female, 145 cases were 70-79 years old, and Valacyclovir was the only drug used in 257 patients.12
Various risk factors associated with acyclovir nephrotoxicity has been remarked by Fleischer in 20191 ie.: hypovolemia, rapid IV acyclovir infusion, using a high dose, concurrent kidney disease and concurrent use of other nephrotoxic agents. Lee EU et al.,13 in 2018 report AKI in 51 of 287 cases (17.8%) who were medicated IV acyclovir and found that there was no significant difference in age, sex, total dose, drug duration and hydration status between AKI and non-AKI group. However, high systolic blood pressure, underlying diabetes, concomitant vancomycin and non-steroidal anti-inflammatory drugs (NSAIDs) use was positively correlated with AKI occurrence. Zhihua Yue14 (2017) found that IV acyclovir had reporting odds ratio (ROR) signal for AKI was 17.68 higher than Valacyclovir, oral acyclovir and famciclovir (ROR 3.97, 3.09 and 2.93 respectively) from the 8,037 reports in FDA AERS database. Although older age may be a risk factor as in the product prescribing information warning, cases in children as young as 13 months old have been reported. The case presented with gross hematuria and crystalluria was found.15,16 AKI that occurs after a single oral dose of Valacyclovir is possible as Unsal MA et al,17 case report in 2018. Higher doses of Valacyclovir may be safe as in Meur YL18 correspondence followed the use of Valacyclovir 6-8 g/day in 6 cases after renal transplantation for prevention of cytomegalovirus and found that after 3 months, there was no increase in the serum creatinine and crystalluria was not detected. Lam NN and team19 carried out another study, which was a matched retrospective population-based cohort study of older adults (mean 77 years) in Ontario, Canada with the outpatient setting of higher (n = 23,256) or lower (n = 3,876) dose of one of three oral antivirals for the treatment of herpes zoster (acyclovir 4,000 vs 800-3,200 mg/day; Valacyclovir 3,000 vs 500-2,000 mg/day and famvir 1,500 vs 350-1,000 mg/day), and found that a higher dose of the antiviral drug, compared to a lower dose, was not associated with an increased risk of hospitalization with an urgent head CT scan, and was not associated with a higher risk of all-cause mortality.19
However, Valacyclovir taken 8g/day, which was seen to significantly reduce the risk of cytomegalovirus (CMV), and was found to be associated with earlier mortality in Feinberg JE20 study (1998). Valacyclovir-related AKI occurred more in the summer as noticed by Inaba I21 (2019) from the Japanese Adverse Drug Event Report (JADER) database and was assumed to be from dehydration. The same study also raised the risk factors to be NSAIDs use, elderly, being female, hypertension and using renin-angiotensin system (RAS) inhibitors. Concomitant use of Loxoprofen, which is a NSAID with Valacyclovir, had an AKI ROR of 26.0 compared to ROR 1.4 when neither drug was used, and ROR 4.6 for when either drug was used. Concomitant NSAIDs use was also reported often from others in Valacyclovir AKI cases.22,23 In this case of Valacyclovir nephrotoxicity showed non-oliguric renal failure as had been reported16 and renal function fairly rapidly improved after patient stopped taking medicine in a good hydration status. In other case reports, oliguric renal failure could occur, and some required more complicated management such as hemodialysis or hemoperfusion.23-25Valacyclovir nephrotoxicity could present along with neurotoxicity, some with electrolyte imbalance, but neurotoxicity could present alone with a reserved renal function.25,26
Valacyclovir nephrotoxicity can present with a wide clinical spectrum, from very mild to very severe, in ages from very young to old, onset from the first dose to 4 days after initiation and sometimes presented along with neurotoxicity. Various risk factors have been reported, but concurrent use of NSAIDs seem to be a consistent suggestion and using RAS inhibitors was also raised. Summer season may explain dehydration status and this was noticed to increase risk. Valacyclovir and acyclovir are now well known antiviral medications, where most feel safe using them, but on occasion, nephrotoxicity can unexpectedly occur. This case report is just another case of Valacyclovir nephrotoxicity to increase awareness when prescribing the medicine.