Breast cancer is one of the most common malignancies in women worldwide. Even though most patients suffer a single lesion, approximately 5-10% of them have multiple tumors, either unilaterally or bilaterally.1,2 Clonal determination of multiple breast cancers in the same patient has been evaluated by multiple techniques, and several lines of evidence have suggested that most cases of unilateral multiple breast cancer (synchronous or metachronous) are clonally-related, whereas the majority of bilateral breast cancers arise from different neoplastic clones.1,3-10 With greater improvements in treating breast cancer in the past few decades, there has been an increasing awareness by both the clinician and the patient to request for biomarkers in order to determine treatment choices. This is particularly true for hormonal receptors (estrogen and progesterone) and for the HER-2 assessment. However, although currently it is recommended to perform these markers in every invasive breast cancer case, as well as in both tumors as in the case of bilateral breast cancer,11 there is no specific recommendation as to whether these biomarkers should be obtained in one or all tumor(s) in patients with unilateral multiple breast cancer.
Based on the evidence that most unilateral multiple breast cancers are clonal-related; it is proposed that analyzing biomarkers on a single lesion is sufficient. This assumption has further been supported by a previous study by Middleton, et al. noting an identical immuno- histochemical profile of synchronous unilateral multiple breast carcinomas, with regard to estrogen receptor (ER), progesterone receptor (PR), and HER-2.3 The purpose of this study was to re-examine the usefulness of biomarker assays in all multiple tumors in the same breast, and to evaluate the genetic heterogeneity of unilateral multiple breast carcinoma.
Case Selection
A series of synchronous unilateral multiple breast cancers was assembled from the Pathology Files at Department of Pathology, Faculty of Medicine, Chulalongkorn University, Bangkok;Department of Pathology, Faculty of Medicine, Chiang Mai University, Chiang Mai;and the National Cancer Institute and the Institute of Pathology, Department of Medical Services, Ministry of Public Health, Bangkok. Formalin-fixed paraffin- embedded tissue was available on 32 tumors from 15 patients. All of the cases met the criteria for synchronous multicentric breast carcinomas.2 Tumors were either 5 cm apart or within the different breast quadrants, with no identifiable connection between lesions, and were diagnosed at the same time for an individual patient. All tumors were classified and graded according to the recent World Health Organization Classification.11
Immunohistochemical Analysis
Immunohistochemical staining was performed on 4μ paraffin sections of all 32 tumors. The antibodies used were directed against ER (Neomarker, Fremont, USA; dilution 1:500), PR (Neomarker, dilution 1:500), and HER-2 (Dakocytomation, dilution 1:500). ER and PR stains were performed by automated immunostainer (Ventana Benchmark LT, Tucson, USA). The HER-2 staining was performed manually by the method described in the HercepTest (Dakocytomation). Immunoreactivity of ER and PR was recorded as positive if ≥1% of the tumor nuclei stained.11 Immunoreactivity for HER-2 was considered to be positive if more than 10% of tumor cells showed intense circumferential membrane staining (3+).12
Fluorescence In Situ Hybridization (FISH) for HER-2 Gene Amplification
Multiple tumors in the same breast with discordant HER-2 immunoreactivity were subject to FISH assay for HER-2 gene amplification (Dakocytomation HER-2 FISH pharmDxTM Kit). HER-2 gene was considered to be amplified when the average HER-2 gene/chromosome 17 ratio was greater than 2.0.12
Microsatellite Analysis
Prior to DNA extraction by MasterPureTM Complete DNA & RNA purification kit (Epicentre, Madison, USA), manual microdissection was performed on paraffin sections to obtain tumor and normal tissue. The latter was derived from nipple skin or axillary lymph nodes of the corresponding case. Ten microsatellite polymorphic markers related to breast cancer on chromosome arms 1p (D1S228), 1q (D1S2878), 3p (D3S1300), 8p (D8S258), 8q (D8S137), 11q (D11S528 and D11S4175), 16q (D16S421 and D16S422) and 17q (D17S800) were used to assess the pattern of allelic loss.1,13-15 Information regarding the cytogenetic localization of the markers was obtained from the Genome Data Base (http://www.ncbi.nlm.nih.gov). One strand of each primer pair was end-labelled, and the PCR reactions performed as described previously.16-17 Microsatellite bands were visualized on a PhosphoImager, using ImageQuanNTTM software (Molecular Dynamics, Sunnyvale, CA). Loss of heterozygosity (LOH) was scored positive when a tumor sample demonstrated reduction of ≥ 66% signal intensity of a microsatellite allele, when compared to the matched normal DNA. Micro- satellite instability (MSI), as defined by the presence of shifted or extra microsatellite allele(s) compared to normal, was also recorded.
Heterogeneity of Unilateral Multiple Tumors
The heterogeneity of tumors from the same breast were considered when they showed different immunohistochemical profiles (at least for one of the markers examined), a difference in FISH results, and/or a different pattern of allelic loss on at least one of the microsatellite markers.
Pathological Findings
All tumors were classified as invasive carcinoma of no special type. The morphological features and histologic grades of tumors were comparable for an individual patient.
Immunohistochemical Analysis
For an individual patient, multiple tumors showed an identical immunohistochemical profile with respect to ER and PR (Table 1). The one exception was case 9 in which the multiple tumors had identical hormonal receptor immunostaining, but different HER-2 immunoreactivity, with one tumor showing 3+ HER-2 and the other showing a negative result (Figure 1).
HER-2 FISH Test
The two tumors in case 9 were evaluated by FISH for HER-2 gene amplification. The differences in HER-2 immunoreactivity between tumors correlated with amplification status of the gene by FISH assay. Amplification of the HER-2 gene was found in one (T2) but not the other (T1), with HER-2 gene/chromosome 17 ratios of 2.3 and 1.1, respectively (Figure 1).
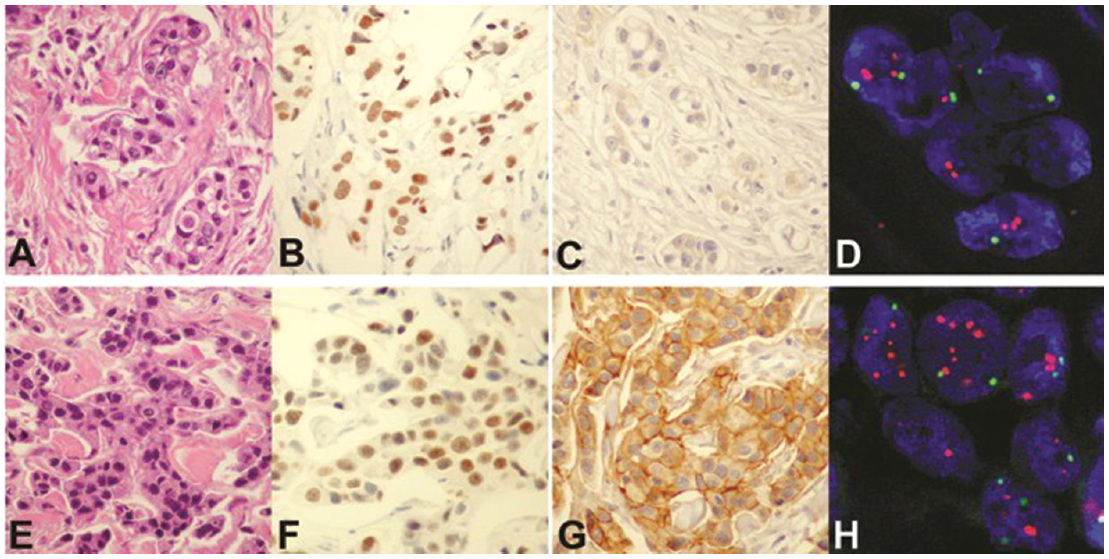
Figure 1: Morphological analysis of unilateral multiple breast cancers. Upper panel from T1 of case 9: Tumor cells (A, hematoxylin and eosin) are positive for estrogen receptor (B) and negative for HER-2 by immunostaining (C), and demonstrate no HER-2 gene amplification (D, FISH, red signals = HER-2 gene and green signals = centromere 17). Lower panel from T2 of the same case: Tumor cells (E, hematoxylin and eosin) are positive for estrogen receptor (F) and HER-2 by immunohistostaining (G), and show HER-2 gene amplification (H, FISH). Both tumors are immunonegative for progesterone receptor and p53 (not shown). (T=tumor)
Microsatellite Analysis
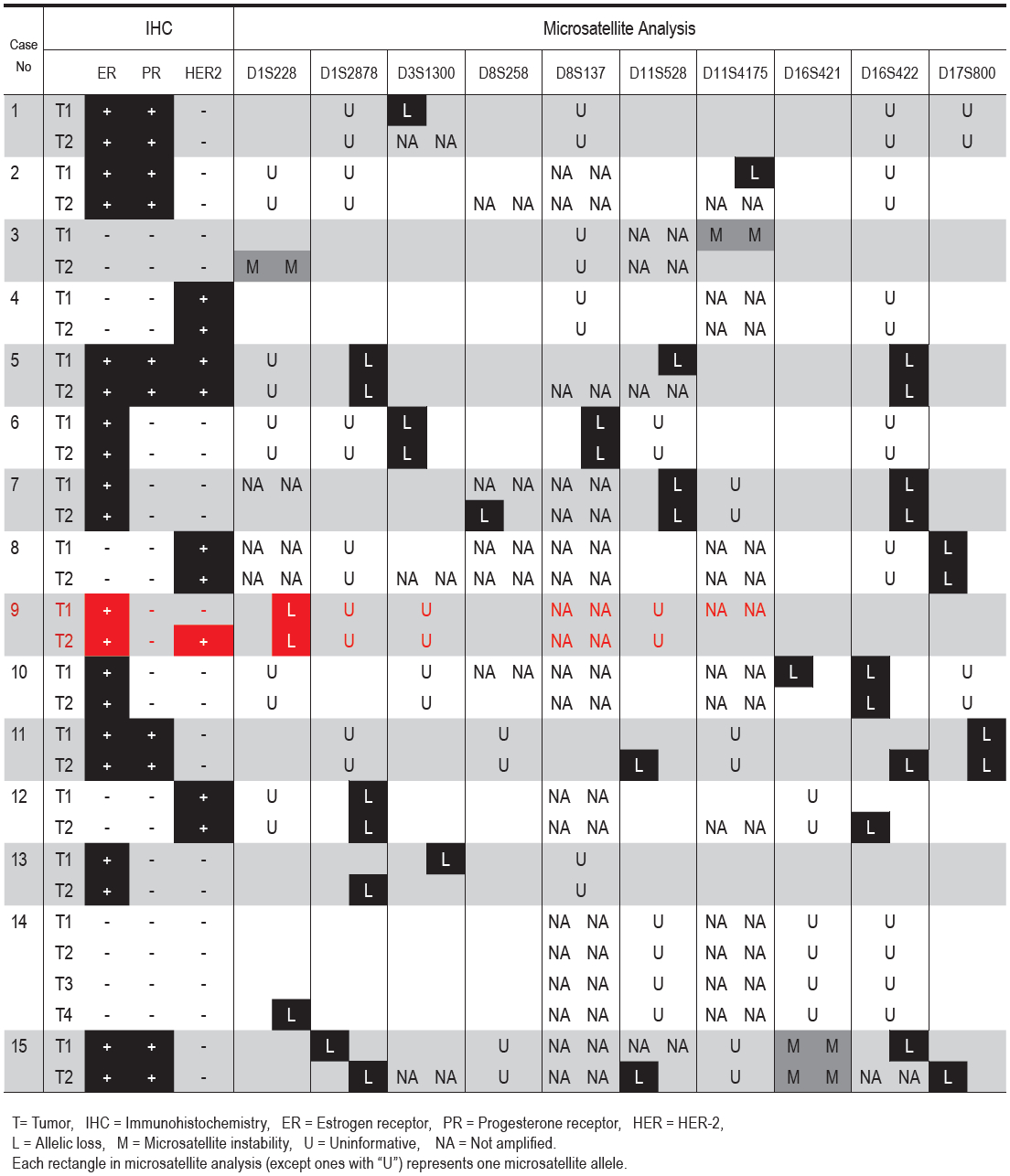
Thirteen cases (86.6%) showed LOH in at least one tumor and for at least one microsatellite locus examined (Table 1). Genetic heterogeneity was observed in 6 cases (cases 10-15) (40%). All of these cases had microsatellite marker(s) that showed allelic loss in one but not the other lesion(s). In case 15, loss of different alleles was observed at one locus (D1S2878). MSI was present in 2 cases (13.3%).
Heterogeneity of Unilateral Multiple Tumors
Based on the criteria outlined above, the heterogeneity of unilateral multiple breast carcinoma was present in 7 cases (46.7%), one (case 9) determined by HER-2 assessment and the remaining 6 cases (10-15) by microsatellite analysis. All tumors in the same breast showed identical immunostaining for ER and PR.
Approximately 5-10% of breast cancer patients have multiple tumors, either unilaterally or bilaterally.1,2 Although multiple lines of evidence have suggested that most unilateral multiple breast carcinomas are clonally-related and likely represent intra-mammary spread of a single lesion in synchronous cases and recurrences in metachronous cases,3-5,7 this does not necessarily indicate that all cancers in the same breast are identical. Middleton et al noted previously an identical immunohistochemical profile of synchronous unilateral multiple breast carcinoma, with regard to ER and PR.3 In contrast, this same study found no differences in HER-2 expression in unilateral multiple breast carcinomas, whereas we were able to establish one patient whose tumors showed different HER-2 status. Identification of the HER-2 gene amplification in one of her tumors played a significant role in the treatment of this particular patient, and the finding may have been missed had only one tumor been examined. In addition, we were able to demonstrate genetic heterogeneity between tumors in the same breast in 6/15 cases (40%) by assessment of the LOH pattern.
The frequency of MSI in breast carcinoma varies considerably, due largely to the lack of well-defined criteria for the assessment. MSI has been observed to be a frequent finding (33%) in advanced breast cancers.18 Not all of our patients had advanced disease, however, so the lower frequency of MSI (13.3%) in our cases is not unexpected. Whether the presence of MSI has an impact upon the outcome of unilateral multiple breast cancer deserves additional study.
Table: Immunohistochemical and microsatellite analyses of unilateral multiple breast cancer.
In conclusion, heterogeneity is commonly observed among tumors in unilateral multiple breast cancer. Even though the differences were minimal with respect to the routinely-used biomarkers (ER, PR, and HER2), more frequent differences were shown at the molecular level. Therefore, all of the present and future prognostic and therapeutic biomarkers should be evaluated in all masses in the same breast, in order to provide the full benefits of treatment modalities to patients with unilateral multiple breast cancer.
This work was supported by grants from the Thailand Research Fund (to SS and AM) and The Pharmaceutical Research and Manufacturers Association of Thailand (to SS). None of the authors have any conflict of interest to declare.