Congenital heart disease has been referred to a congenital defect of the heart structure that may be a common or a rare type. The rare type corre-sponds to just a few percent in the incidence rate when compared to other congenital heart diseases. Each congenital heart disease has its own special charac- teristics for each specific type of diagnostic images. Magnetic Resonance Imaging (MRI) is the most successful noninvasive tool in comprehensively demonstrating the pathological structure of simple or complex congenital heart disease and is able to visualize some abnormalities that echocardiography has failed to do. In addition MRI has evolved into the gold standard in many techniques for CHD diagnosis such as congenital vessel anomaly.1 As mentioned above; the cardiac imaging specialist may diagnose the disease on first sight. The most important issue for successful diagnosis of CHD regardless of the imaging tools used is to transform the knowledge of main key pathologies of disease to be displayed in a substantial fashion on a diagnostic image. Therefore the comprehensive knowledge of the key pathologic characteristics of the CHD cases of interest and imaging techniques are important elements for imaging protocol planning to obtain the diagnostic images required. The comprehensive strategies to obtain key images in diagnosing CHD especially in the unseen cases are: 1) Get a clue from the definition of the disease such as an atrial septal defect that is referred to the defect of the inter-atrial septum, therefore we must operate the imaging tool in such a technique and plane that the inter-atrial septum will be clearly visualized. This image technique and the recommended plane on MRI is gradient echo CINE technique on cardiac four-chamber plane or may be the spin echo technique on four chamber plane; 2) Read about the associate diseases in both intra- and extra- cardiac types and transform these associate abnormal structures to show on the image and; 3) Study each imaging technique of each imaging tool and learn how to use it properly including how to select the proper cardiac plane. However, please note that the root cause of success comes from truly knowing the characteristics and position of the cardiovascular anatomical structure on any standard and cardiac plane on any imaging tool. The physiologic change of the function, contour and blood flow direction in any cardiac chambers and great vessels always occurs as a consequence of congenital anomalies. Hence, to create perfect diagnostic images you need to use techniques that are able to display the defect anatomy and physiologic changes including the signal void of blood flow direction in the same image. The gradient echo CINE MRI on axial view with whole cardiac contour and great vessels coverage in all anterior-posterior, superior –inferior, right to left directions is recommended as a first scout and it may be used as a diagnostic image. Other MRI pulse sequence techniques may be used to confirm the diagnosis or in complementary diagnosis. The Spin echo MRI technique can provide the anatomical structural images but cannot display physiologic change information. The principle of sequential segmental analysis approach for CHD is recommended as a guiding tool for perfect CHD approach as an imaging helping tool.2
Coronary sinus ASD or the unroofed ASD has been reported in about less than 1% of cases compared to all ASD.3 This ASD type is not a defect of the atrial septum but is caused by the absence of the wall that separates the coronary sinus from the left atrium.4 This characteristic is the diagnostic clue of coronary sinus atrial septal defect. The coronary sinus ASD is usually associated with a persistent left superior vena cava (PLSVC), which is the most common anomaly of systemic venous drainage.5 The coronary sinus is the anatomical structure that is running transversely at the posterior of the left atrio-ventricular groove of the heart. The coronary sinus collects the blood from the great cardiac veins and it is emptying blood into the right atrium. The key pathologic defect of this type of ASD is a defect hole that communicates the coronary sinus and the left atrium. The multi-slices images of gradient echo CINE MRI on transverse or axial plane with whole cardiac contour and great vessels coverage is recommended as a scout image (see Figure 1.1). By these scout images all the cardiac chambers, great vessels, signal void of normal and abnormal blood flow direction including associated diseases such as PLSVC can be visualized. PLSVC is seen at the left lateral of the aortic arch. The defect between the coronary sinus and left atrium is visualized on the axial view and it can be confirmed by using the gradient echo CINE MRI technique on short axis plane of the atria that is cut parallel through the coronary sinus course (see Figure 1.2). The persistent left superior vana cava (PLSVC) can be demonstrated in a whole course by a first pass MR angiography with gadolinium contrast injection. The gradient echo Q flow mapping MRI on short axis view of the atrium that is cut parallel through the course of the coronary sinus is used to displayed the flow across the communication defect between the left atrium and the coronary sinus (see Figure 1.3-1.4).
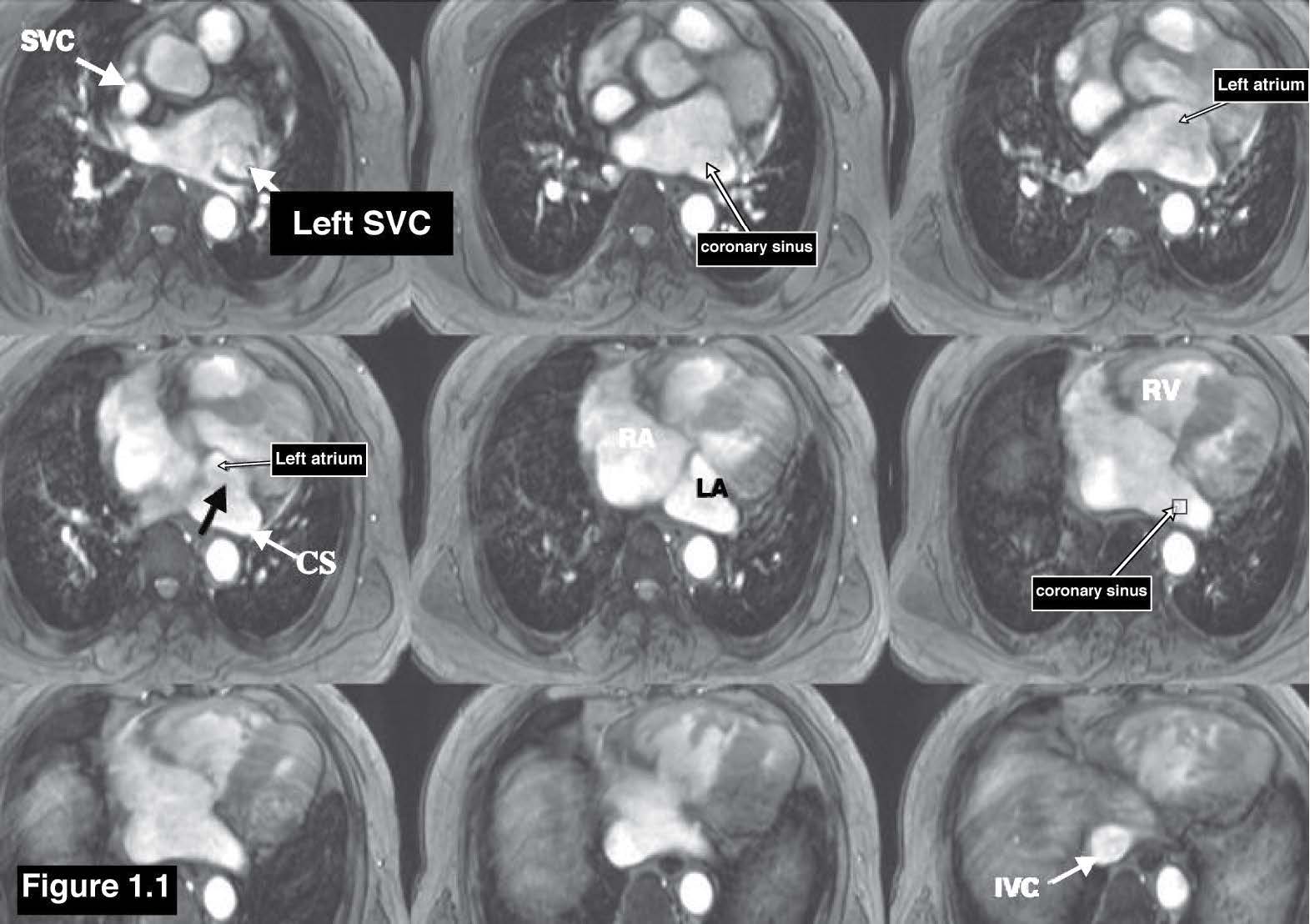
Figure 1.1: Coronary Sinus ASD case: The scout images of the gradient echo CINE MRI on axial view demonstrate the contour of all cardiac chambers and also display the communicating defect between the left atrium and coronary sinus (black arrow sign ) , CS= coronary sinus , SVC= Superior Vena Cava , IVC= Inferior Vena Cava.
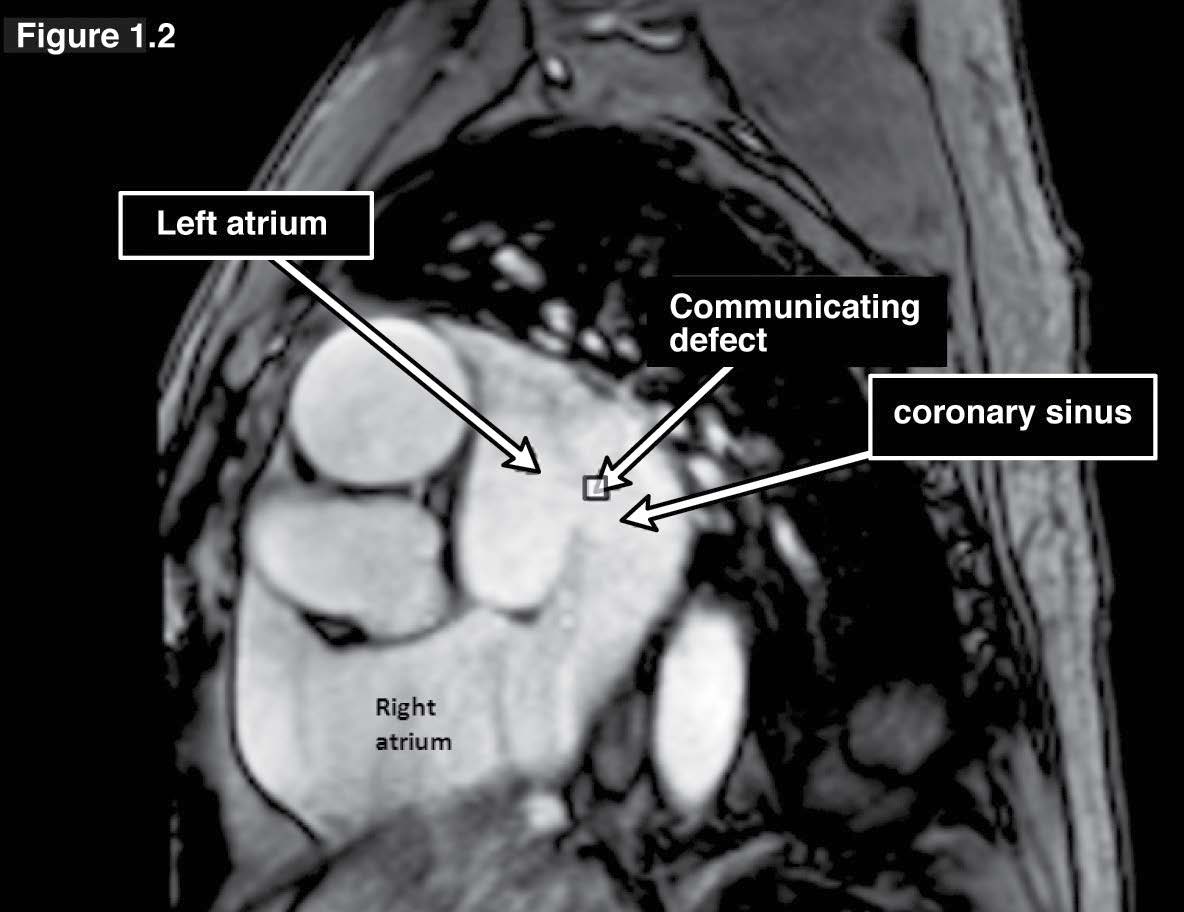
Figure 1.2: Coronary sinus ASD case: The gradient echo CINE MRI on short axis view of the atria on the slice cut parallel through the coronary sinus course demonstrates the communicating defect of the coronary sinus and the left atrium.
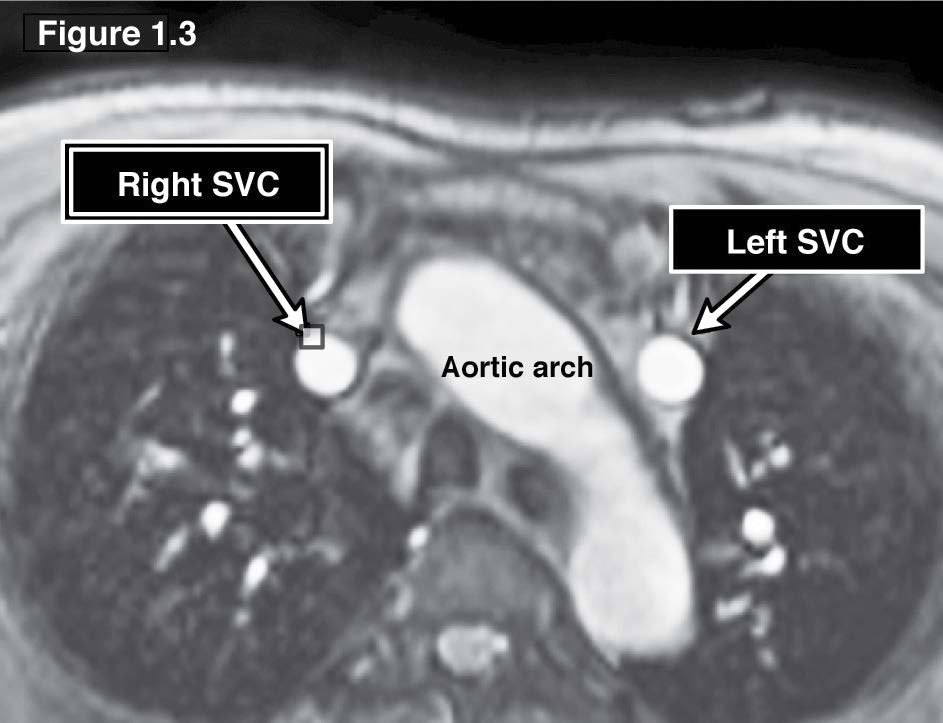
Figure 1.3: Coronary sinus ASD case: The gradient echo CINE MRI on axial view scout image demonstrates the persistent left SVC (PLSVC) at the left lateral side of the aortic arch.
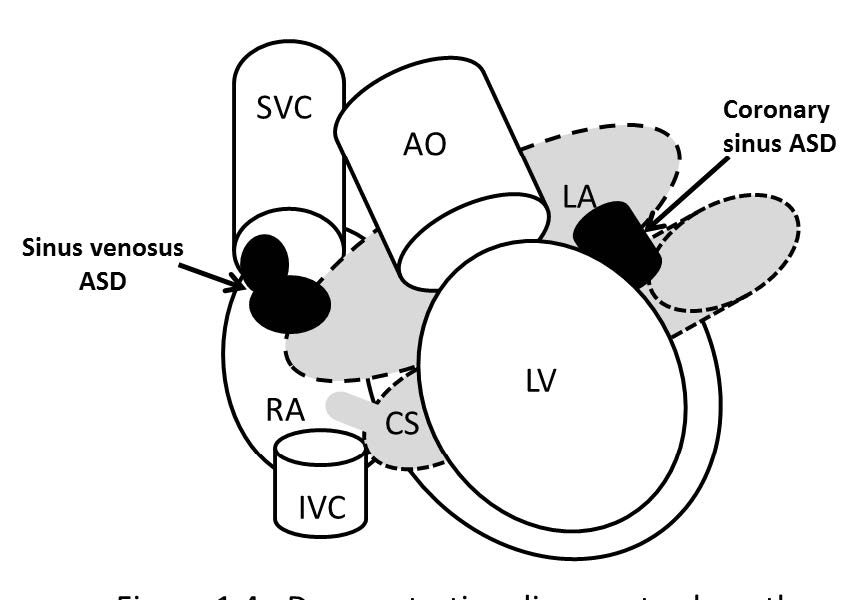
Figure 1.4: Demonstrating diagram to show pathologic defect of si- nus venosus ASD and coronary sinus ASD.
Sinus venosus ASD accounts for 10% of all atrial septal defects 6 It is the second rarest type of ASD. The defect lesion causes the communication as a left to right shunt between the great vein (Superior vena cava (SVC) or Inferior vena cava (IVC)) and the left atrium through the defect of the inter atrial septum. The most common defect lesion of the inter atrial septum is at the upper atrial septum and posterior to the fossa ovalis. The almost always associated condition is partial anomalous pulmonary venous drainage (PAPVD) of the right upper lung pulmonary veins to the SVC.7 To identify this anomaly is to demonstrate the structural communication defect of the SVC or IVC and the left atrium. As is well known, the structural contour of these two vena cava vessels are in normal circumstances straight standing parallel to the spine axis above and below the right thoracic diaphragm respectively. The recommended MRI scout image on axial plane is the best plane to identify the round shape vena cava structure and its border. To identify the round shape border of the vena cava at the emptying port into the right atrium (RA) is an easy first step to approach this disease on MRI. The defect of the round shape of vena cava at the emptying port into the RA is a suspicious clue and when combined with the defect of the upper septum the diagnosis is obtained. Four- chamber or horizontal plane on MRI may not be the appropriate diagnostic used plane because the vena cava contour will be distorted and it is difficult to identify its structure and the correlation structure. On the axial plane of the gradient echo CINE MRI, the occurrence of PAPVD whether at the SVC or the IVC can be visualized. However, MR angiography with gadolinium contrast injection is still an important technique to identify the whole running course of the PAPVD (see Figure 1.3,2.1-2.3).
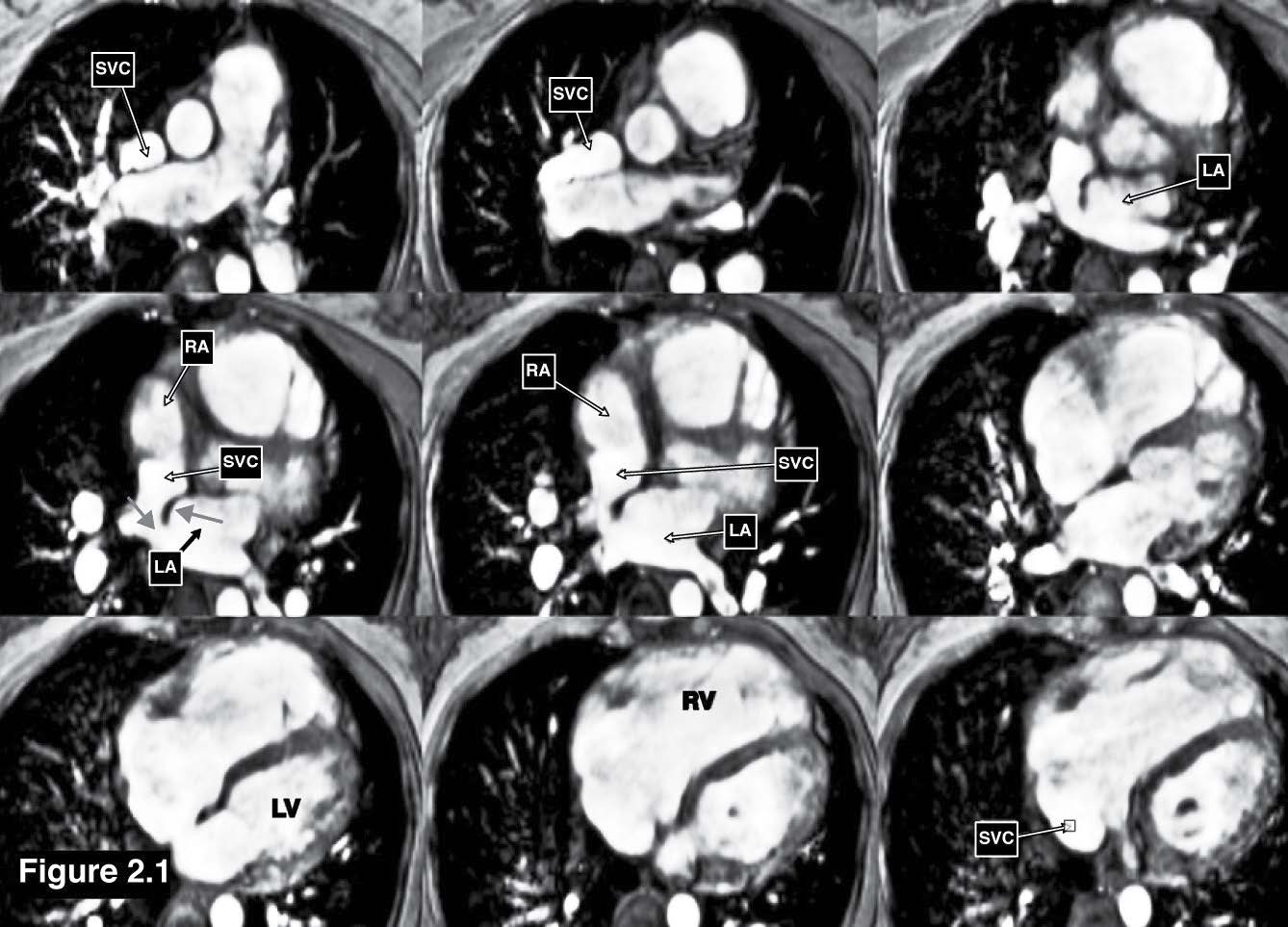
Figure 2.1: Sinus venosus ASD case: The gradient echo CINE MRI images demonstrate the cardiac chamber contour and also display the communicating defect between the left atrium and the SVC through the defect inter-atrium septum (red arrow sign). RA= right atrium , LA= left atrium , RV= right ventricle, LV = left ventricle.
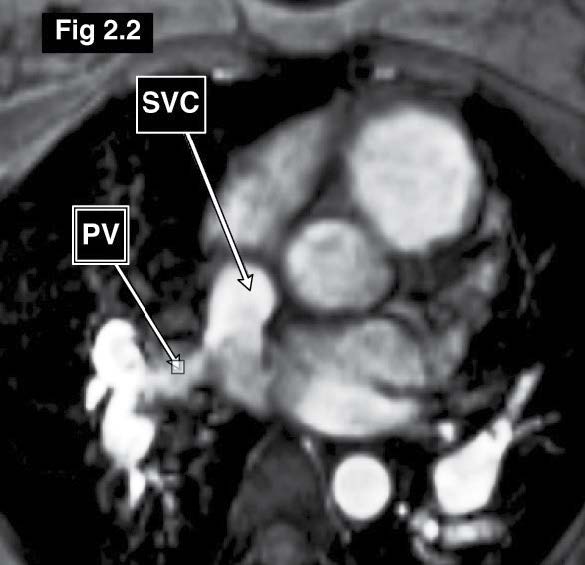
Figure 2.2: Sinus venosus ASD case: The gradient echo CINE MRI on the axial plane demonstrates the drainage of the blood flow from the right upper lung pulmonary veins into the right SVC (PAPVR).
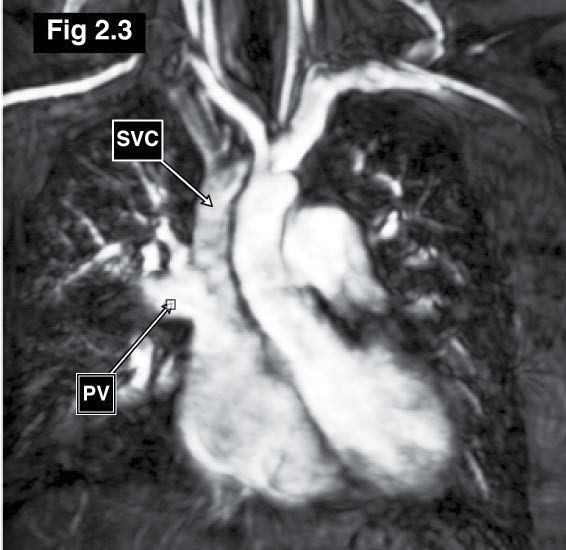
Figure 2.3: Sinus venosus ASD case: The MRA (angiography) with gadolinium contrast injection of great vessels reveals the course of drainage of the right upper lung pulmonary veins into the emptying portion of the right SVC.
Congenital atrial-appendage aneurysm especially of the right atrium is very rare. Only 50 cases of congenital atrial-appendage aneurysm are reported in the literature in 2005.8 The atrial appendage aneurysm whether the left or right atrium is affected can cause the complications such as arrhythmia and thromboembolism. To diagnose the atrial appendage aneurysm on MRI the whole contour of both atria and their appendages must be demonstrated. Scout scanning using the gradient echo CINE images on axial plane, will identify the left (LAA) and right (RAA) atrial appendage. The LAA is a windsock liked structure and is remnant of the embryonic left atrium and it is the closed lateral portion that can be differentiated from the superior pulmonary vein structure. The right atrial appendage is a flat, triangular structure that contains pectinate muscle bundles and it is running parallel to the right atrium.9 The other best MRI technique is the gradient echo CINE MRI on horizontal view with multi-slices for whole atria coverage from anterior to posterior and no gap between the two adjacent slices. Both left and right atrial appendages are visualized. For the left atrial appendage, the gradient echo CINE MRI on vertical long axis view is an another technique to be used to demonstrate the left atrium and its appendage. The MRA (angiography) with first pass contrast injection MRI technique with delayed contrast enhanced technique can be combined and used to identify clots in the atrial appendage. The atrial appendage aneurysm is not a complicated case but is rare and is usually misunderstood as a cardiac or mediastinal mass (see Figure 3.1-3.3).
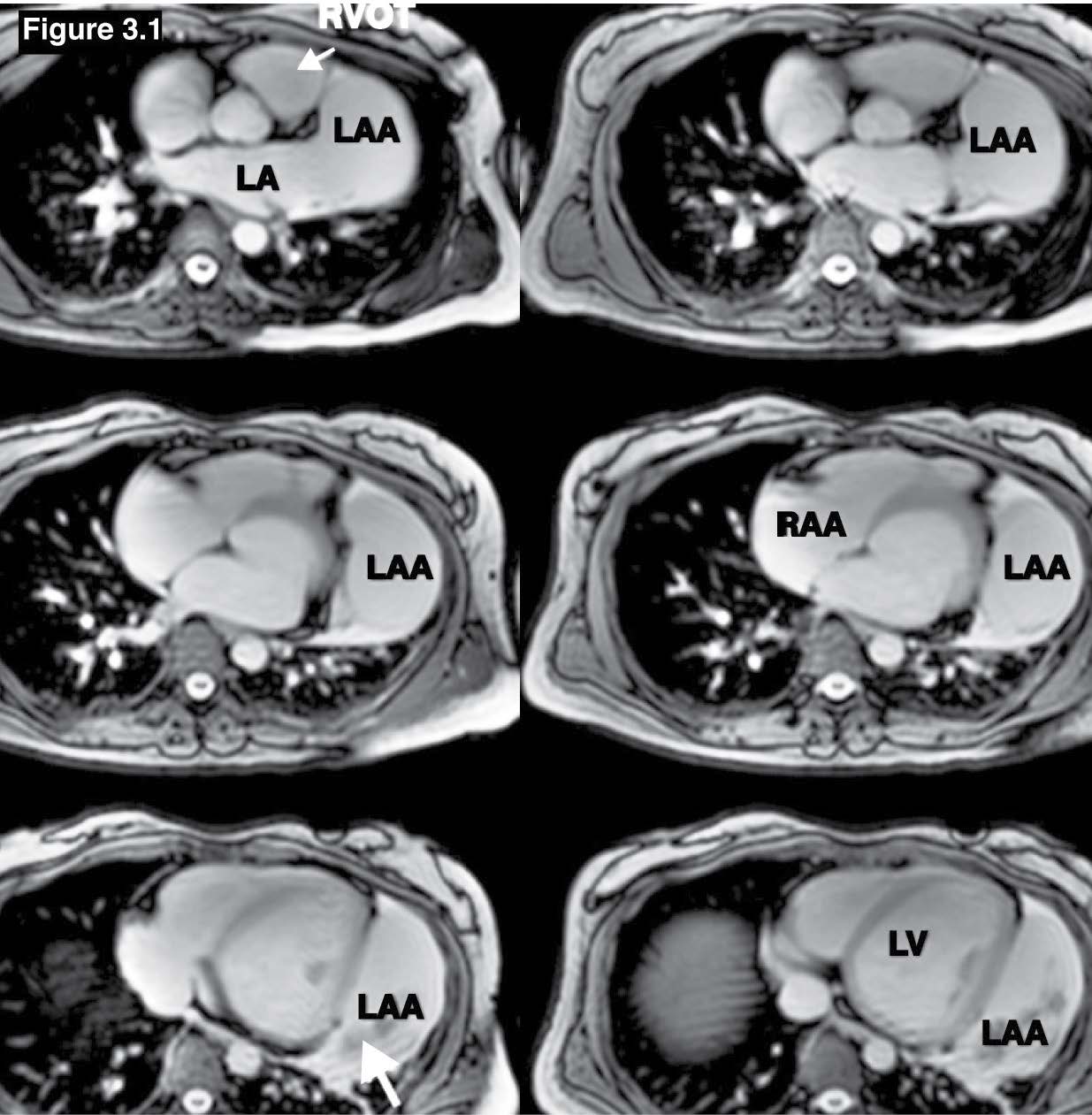
Figure 3.1: Atrial appendage aneurysm case: The gradient echo MRI on axial view shows the huge LAA with some blood clots (Hypo-signal mass, white arrow sign). LAA = left atrial appendage, RAA= right atrial appendage.
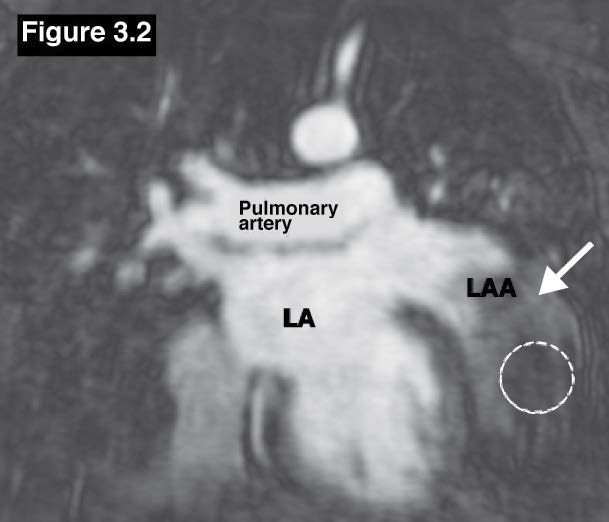
Figure 3.2: Atrial appendage aneurysm case: The MR with delayed contrast enhancement study confirms the LAA aneurysm with clot (dashed circle).
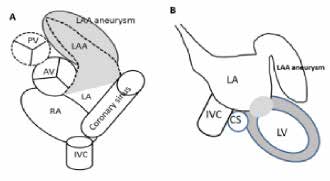
Figure 3.3: A; Demonstrating diagram on short axis view of both atria to show the pathologic left atrial appendage aneurysm. B; LAA aneurysm is shown on coronal view of the heart. AV=aortic value, PV=pulmonic valve, LA=left atrium, RA=right atrium, LAA=left atrial appendage, IVC=inferior ena cava.
Coronary artery fistula (CAF) is a type of congenital coronary anomaly. The anomaly of coronary artery may occur at the origin, the course and the termination site. CAF is a congenital anomaly of the coronary artery termination. There is an abnormal communication track between the coronary artery and the chamber of the heart or any location in the pulmonary circulation (coronary arterio-venous fistula).10 There are terms for calling the specific type of coronary fistula; coronary-cameral fistula is named for the fistula ending at any cardiac chambers, coronary arteriovenous fistula is for the fistula ending at any segment of the systemic or pulmonary circulation system and the term coronary arterial-venous fistula is generally used. In the case of ALCAPA (Abnormal Left Coronary Artery from the Pulmonary Artery) in which the origin of the coronary artery originates from the pulmonary artery is classified as anomalous origin of the coronary artery from the pulmonary artery.11 MRA for coronary artery is the technique of choice for using MRI to identify the coronary artery structure. This technique does not use the gadolinium contrast injection. But however, gradient echo CINE MRI is still an alternative technique of choice to diagnose coronary artery fistula, especially in depicting the termination chamber by detecting the signal void of emptying flow. The advantage of the gradient echo CINE MRI is to provide both anatomical and physiologic changes on the images including the abnormal signal void of the abnormal flow. The key step to identify the coronary fistula is to identify the origin, its course and the termination site. Coronary arterio-venous fistula can be diagnosed whenever there is a connection between the artery and vein (that is not an ALCAPA case). Fistula coronary is usually normal in diameter size and does not cause symptoms. This article shows the left circumflex coronary artery fistula that empties into the right atrium. We begin with the scout investigation the origin of coronary arteries, its running course and termination site by using of gradient echo CINE MRI on axial and the coronal view that covers the whole heart contour and great vessels in all directions. The wrong termination site is found by detecting the emptying flow at the distal LCX into the RA that causes the coronary arterio-venous fistula. An aneurysm is also found at the most distal LCX closed to the fistula region. In the case of small fistula coronary artery, the MRA technique without contrast for coronary artery should be used as a diagnostic technique complimented with the gradient echo CINE MRI technique (see Figure 4.1-4.3).
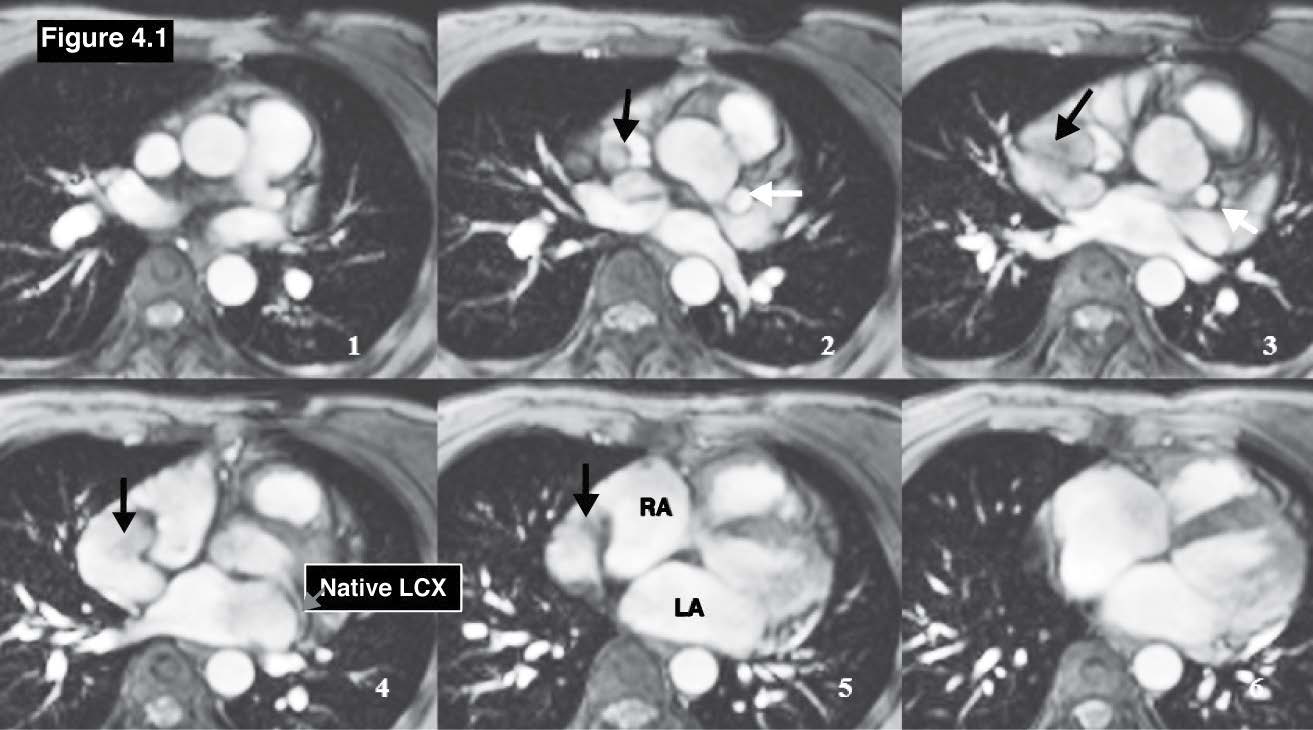
Figure 4.1: Coronary venous fistula case:The gradient echo CINE MRI images on coronal plane demonstrate the running course of the anomalous LCX coronary that originates from the left aortic cusp and running above the roof of the left atrium (LA) and emptying into the RA (black arrow). RA= right atrium, LA= left atrium, LV= left ventricle.
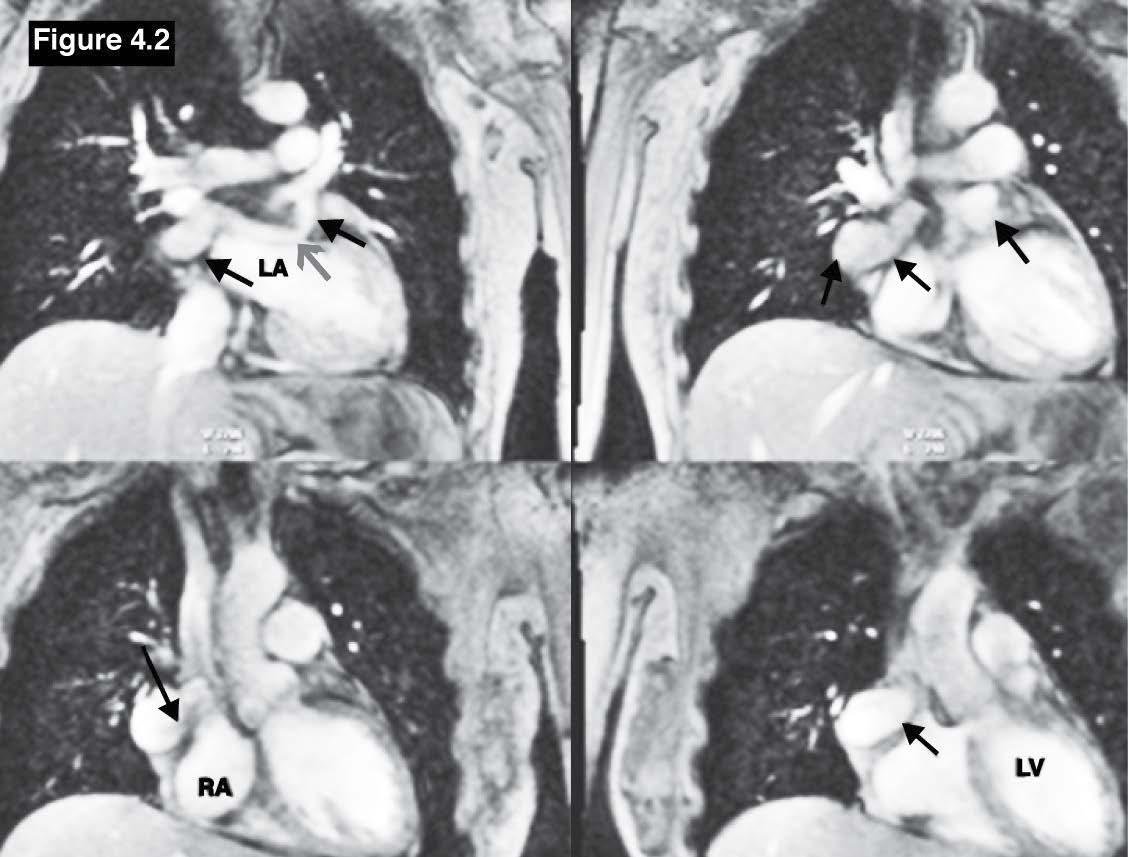
Figure 4.2: Coronary venous fistula case: The gradient echo CINE MRI images on coronal plane demonstrate the running course of the anomalous LCX coronary (red arrow sign) that originates from the left aortic cusp and running above the roof of the leftatrium(LA) and emptying into the RA (black arrow), RA= right atrium, LA= left atrium , LV= left ventricle.
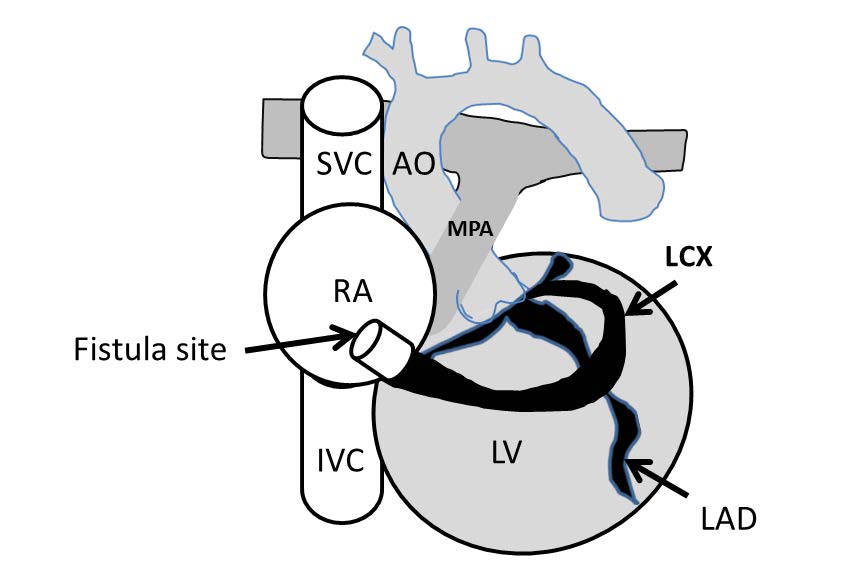
Figure 4.3:The demonstrating diagram of left circumflex coronary fistula (arteriovenous fistula) with emptying into the RA. AO=aorta, MPA=main pulmonary artery, RA=right atrium, LV=left ventricle, SVC=superior vena cava, IVC=inferior vena cava, LAD=left anterior descending cornary artery, LCX=left circumflex coronary artery.
Ebstein’s anomaly is a rare congenital heart disease that accounts for 1% of all congenital heart disease.[12] Ebstein’s anomaly involves the low lying of the tricuspid valve leaflets. The cardinal symptoms of Ebstein’s anomaly are cyanosis, right-sided heart failure, arrhythmias, and sudden cardiac death.13 The key pathology of Ebstein’s anomaly is the apical displacement of the one or more tricuspid leaflets from the tricuspid annulus downward to the apical right ventricle (RV). Ebstein’s anomaly commonly involves the septal leaflet either in isolation or in association with the displacement of the anterior or posterior leaflet. This abnormal displacement creates the portion that is called atrialization and it causes a small true right ventricle. The atrialization portion steps into the part of the true right ventricle (RV) but does not perform a pumping function as RV but it functions as part of RA. Ebstein’s anomaly has developed during the embriological period by the failure of the development of the tricuspid valve and the chordae in reaching the atrio-ventricular (AV) junction and also the failure to complete the resorption of the apical tissue of tricuspid valve.14 Ebstein’s anomaly may be associated with other congenital structural heart diseases including congenital abnormal cardiac electrical conduction system Wolf Parkinson White syndrome. The atrialization of the tricuspid leaflet causes tricuspid regurgitation with varying severity. The severity of TR depends on how far the distance is between the AV junction to the displaced tricuspid septal leaflet. In addition, blood clot formations in the right atrium may occur because the atrailized portion in the RV functions as the resting RV hence blood stagnation can happen in RA because of the discordant contraction between the usual RA and the atrialized RA portion. The pathologic characters of Ebstein’s anomaly are 1) low lying of the septal leaflet; 2) apical displacement of the functional annulus; 3) dilation of the “atrialized” portion and true tricuspid annulus; 4) tethering of the anterior leaflet of the tricuspid valve. Ebstein’s anomaly is suspected whenever the separation distance between the mitral and tricuspid plane is greater than 1 cm. The diagnostic criteria of Ebstein’s anomaly is the downward displacement of the septal and posterior leaflets in relation to the anterior mitral valve leaflet is >8 mm/m2 body surface area. [15] In cases of Ebstein’s anomaly the MRI images in horizontal long axis view (4-chamber view) of gradient echo CINE image is the best technique and plane in which the atrialized displacement of the septal and posterior leaflet of the tricuspid valve is clearly visualized and is easy to measure the distance of downward displacement of the septal leaflet of tricuspid valve in relation to the anterior mitral valve leaflet (See Figure 5.1-5.2).
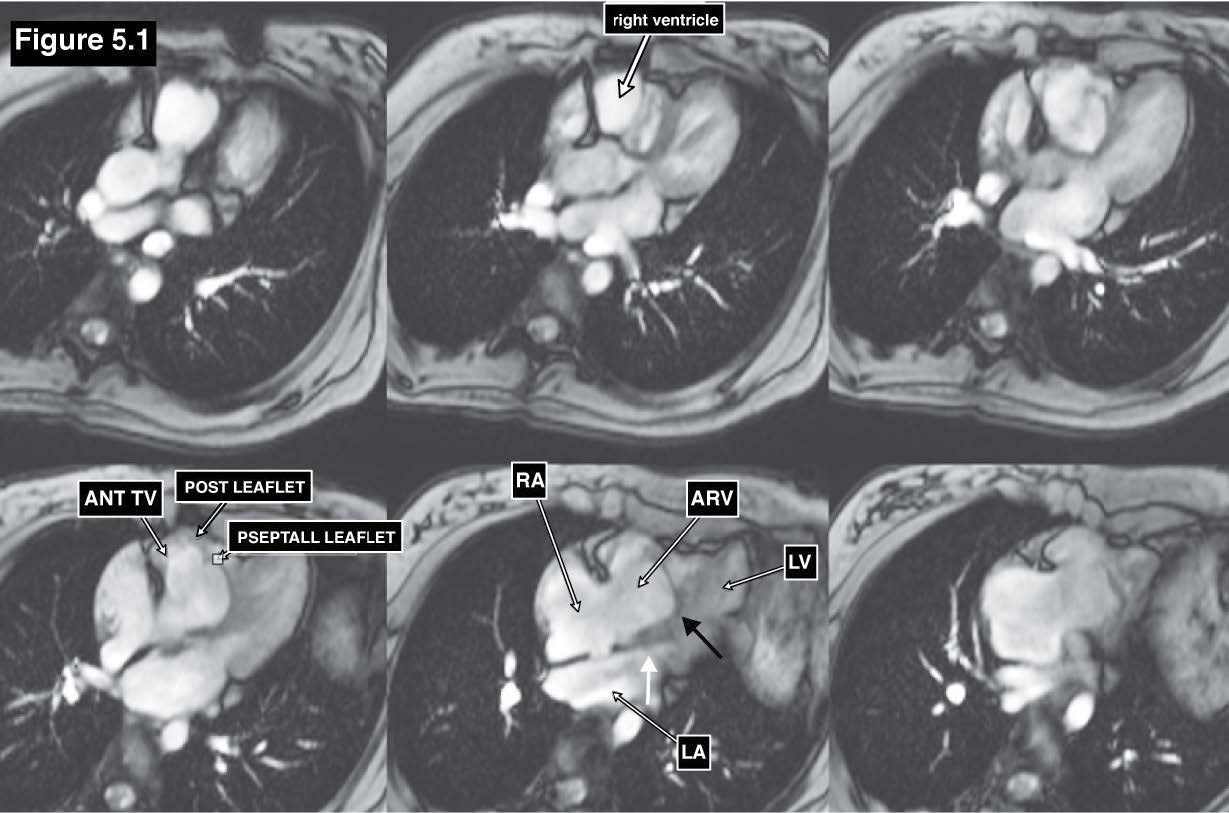
Figure 5.1: Ebstein’s anomaly case: The gradient echo CINE MRI on four-chamber view demonstrates the downward displacement of the septal and posterior leaflets (black arrow) in relation to the anterior mitral valve leaflet insertion (white arrow ) is >8 mm/m2 body surface area of Ebstein’s anomaly.
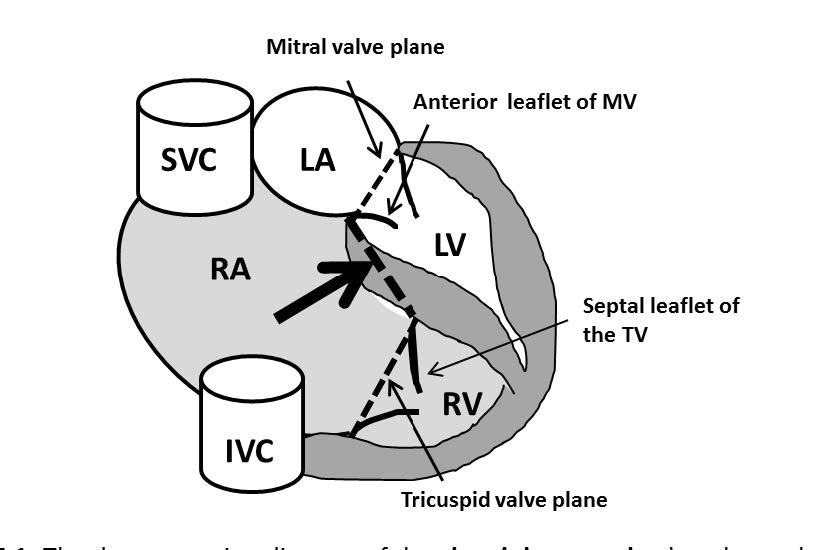
Figure 5.2: The demonstrating diagram of the ebstein’s anomaly that shows the low lying or the downward displacement of the tricuspid valve leaflets. The ebstein’s anomaly will be diagnosed if the downward displacement of the septal and posterior leaflets in relation to the anterior mitral valve leaflet id > 8mm/m2 body surface area. SVC= superior vena cava, IVC= inferior vena cava , LA= left atrium, RA= right atrium , LV= left ventricle , RV= right ventricle , TV= tricuspid valve.
Gerbode defect or congenital left ventricular to right atrial (LV-RA) shunt is a rare congenital heart disease with an incidence <1% of all congenital heart defects.16 The congenital LV-RA shunt less typical than the acquired type. The mechanism of the acquired type is the injury of the membranous septum due to any causes such as infective endocarditis that invades the membranous septum or the tear of the tricuspid annulus at the membranous septum, etc. 17,18 The diagnosis of the Gerbode defect using imaging tools is very challenging because the high jet flow from the LV through the membranous septum to the RA may be misdiagnosed as an isolated tricuspid regurgitation or associated with membranous ventricular septal defect. The key pathologic characters of the Gerbode defect is the shunting defect of blood flow from the LV to RA. The defect is above the septal commissure of the tricuspid valve and below the commissure of the mitral valve. Therefore, the key images on MRI is gradient echo CINE on a four-chamber view that will show the membranous track and the jet flow from the LV into the RA cavity from left to right.
The CINE four-chamber view images should be operated in multi-slice images that cover the anterior to inferior part of the heart because the shunt flow from the LV to the RA may run through in any region from the anterior to inferior of the membranous portion and the axis of the tricuspid valve annulus is not in the right angle to the membranous portion. The coronal view of the heart with whole heart coverage from anterior to posterior direction can be used to confirm the high velocity jet flow position and direction of the LV-RA shunt. In addition, there is an indirect LV-RA shunt that blood flows from the LV to RV by true ventricular septal defect (VSD) and goes through the RA by the defect hole of the septal leaflet of tricuspid valve. Therefore, two jet flows are seen, the first one is in the RV cavity perpendicular to the membranous septum, the second one is the jet flow into the RA perpendicular to the membranous septum as shown in this article. In addition the cardiac valvular structure must be assessed to depict infective endocarditis to rule out acquired LV-RA shunt19 (see Figure 6.1-6.4).
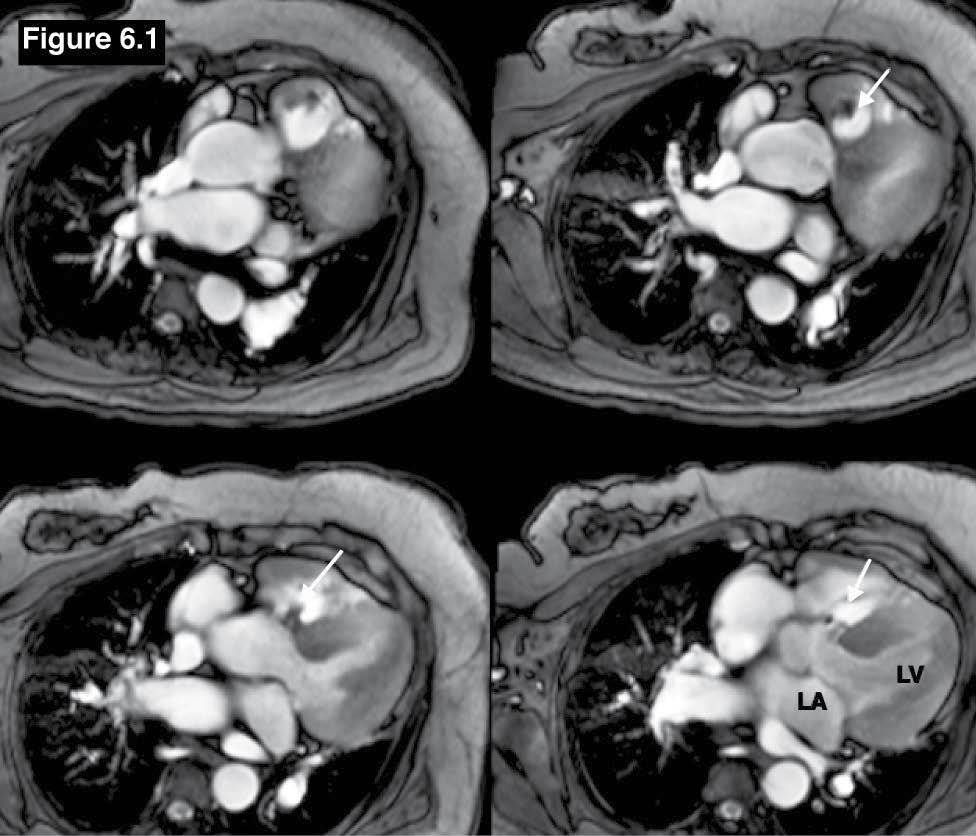
Figure 6.1: Gerbode defect case: The gradient echo CINE MRI on the axial plane images demonstrate a high possible left to right membranous VSD jet flow in a suspicious case of Gerbode defect. Ao=Aorta, LA=left atrium, LV=left ventricle. It has to be confirmed that the left to right VSD flow is going to the RA directly or through the defect of the septal leaflet of the tricuspid valve.
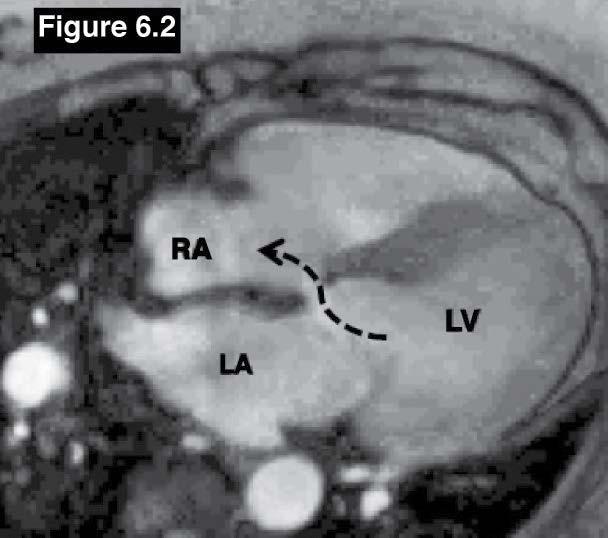
Figure 6.2: Gerbode defect case: The gradient echo CINE MRI on a four-chamber plane image shows the left to right shunt flow from the left ventricle to the right atrium.
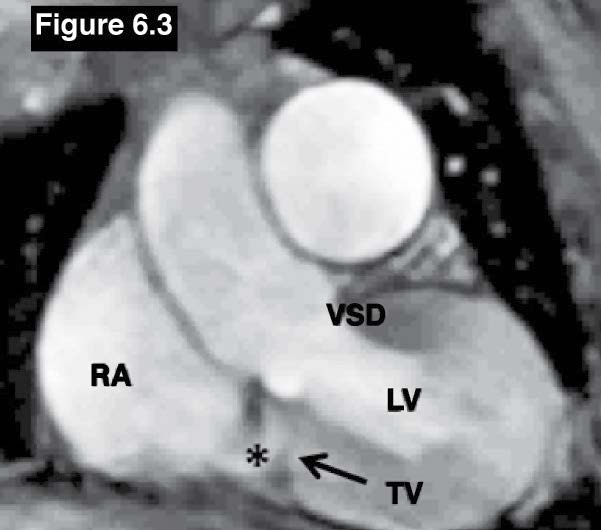
Figure 6.3: Gerbode defect case: The gradient echo CINE MRI on coronal view confirms the left to right flow membranous VSD to the RA(*). RA = right atrium, LA= left atrium , LV= left ventricle, TV=tricuspid valve.
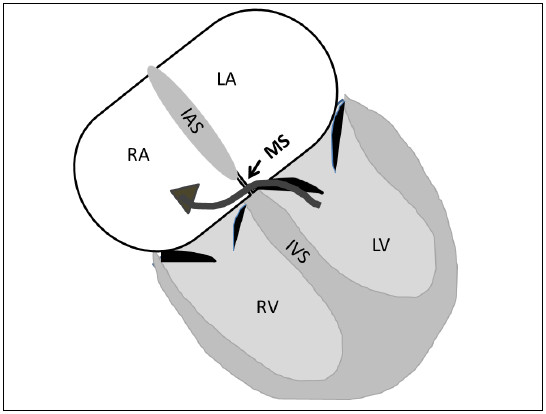
Figure 6.4: The demonstrating diagram of the Gerbode defect or congenital left ventricular to right atrial (LV-RA) shunt. The left to right shunt flow from the left ventricle (LV) to right atrium (RA) is running through the small area of the membranous septum. The potential communicating defect is above the septal commissure of the tricuspid valve and below the commissure of the mitral valve. IAS=inter-atrial septum, MS=membranous septum, IVS=inter ventricular septum.
The persistent left superior vena cava is the result of the failure of the regression of the cardinal vein during normal fetal development. It is a rare case with a 5% incidence of the CHD population.20 Most of the persistent left SVC is draining into the RA and about 10% of the persistent left SVC population is draining into the left atrium results in right to left shunt. The left persistent left SVC stands on the left side of the aortic arch before running anteriorly to the hilum. The key steps to assess persistent left SVC are 1) to identify the existing of the persistent left SVC; 2) the running course and; 3) the termination site. As mentioned above regarding the origin and running course of the LSVC, a routine scout using multi-slice gradient echo CINE images on axial view with whole heart contour and great vessels coverage in all directions should be used as scout images as recommended. Both right and left SVC will be visualized at the right and left lateral site of the aortic arch. Then the running course of the SVC must be tracked to find out the termination site. This article shows the persistent left SVC that runs into the right atrium through the coronary sinus. The existence of the left SVC can be confirmed by using MRA with gadolinium contrast injection of the SVC in first pass and delayed phase of contrast injection (see Figure 7.1-7.3).
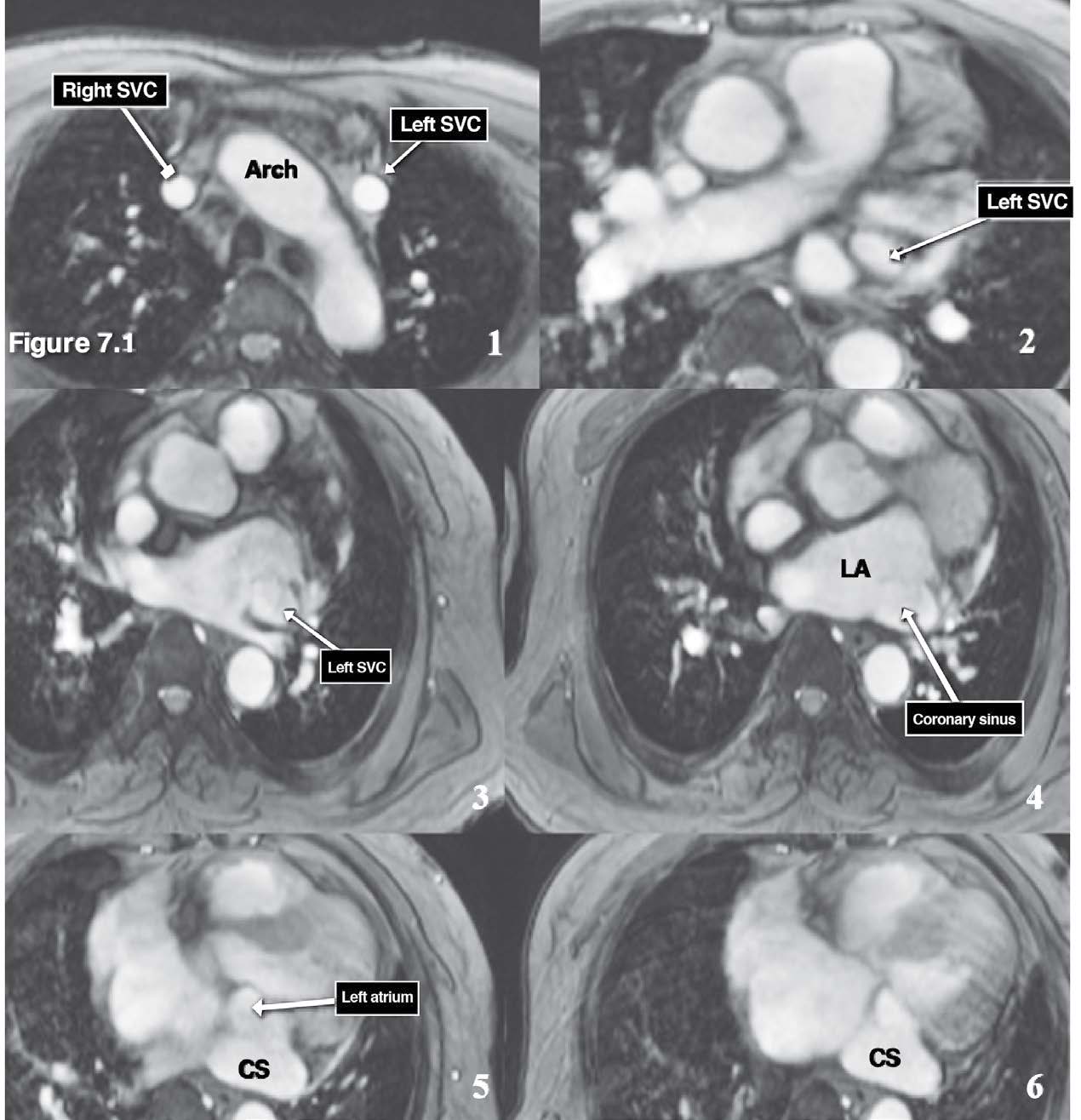
Figure 7.1: Persistent left SVC (PLSVC) emptying into the coronary sinus case: The serial images (1-6) of the gradient echo CINE MRI on axial plane reveal the running course from the origin (left lateral to the aortic arch) to the termination site which is coronary sinus. Arch=aortic arch, SVC= superior vena cava, CS= coronary sinus, LA= left atrium.
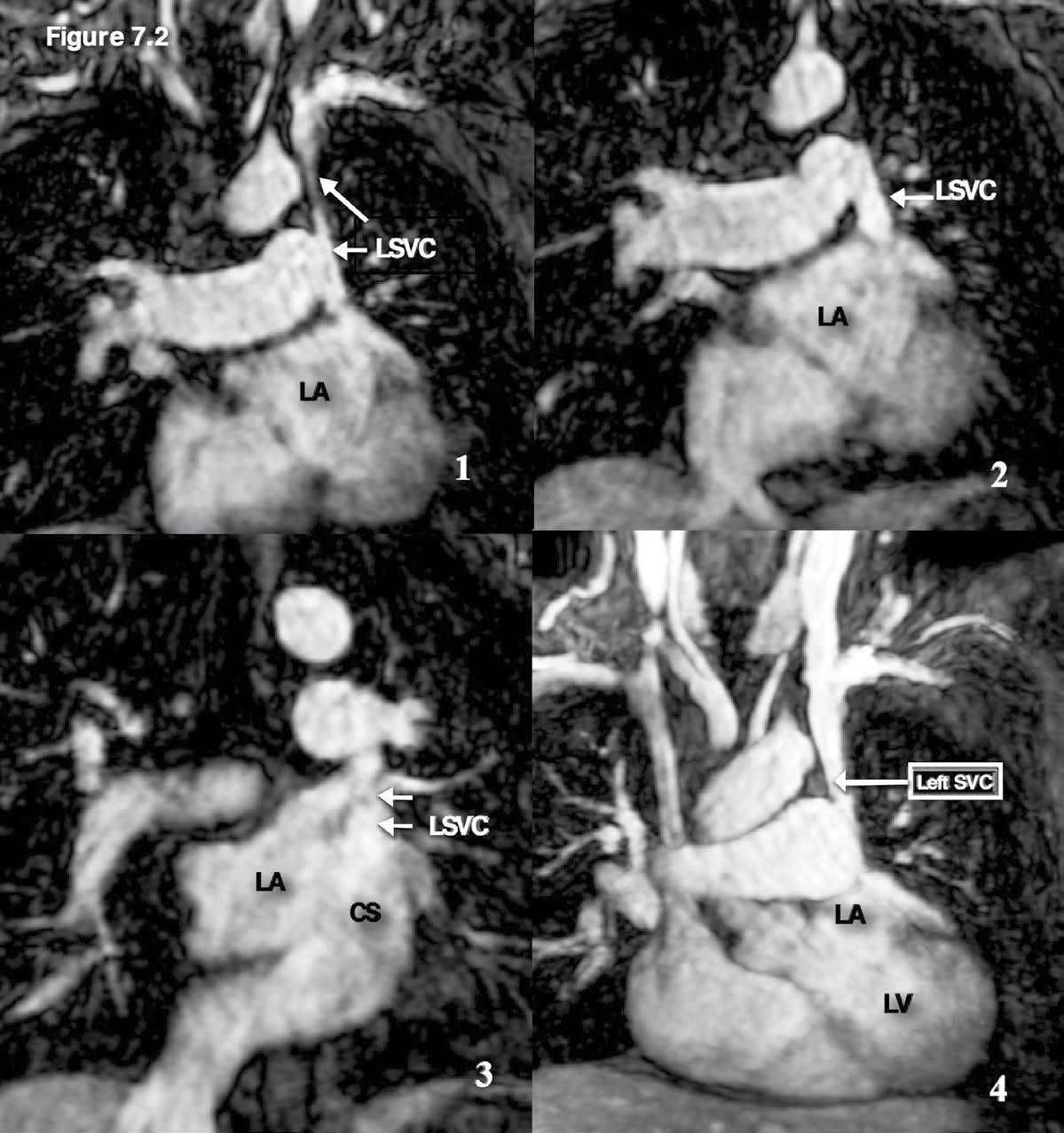
Figure 7.2: Persistent left SVC emptying into the coronary sinus case: The serial images of the MR angiography with gadolinium contrast injection confirm the existence of the left SVC and its termination site is coronary sinus.CS= coronary sinus , LA= left atrium, RA= right atrium , LSVC =left superior vena cava.
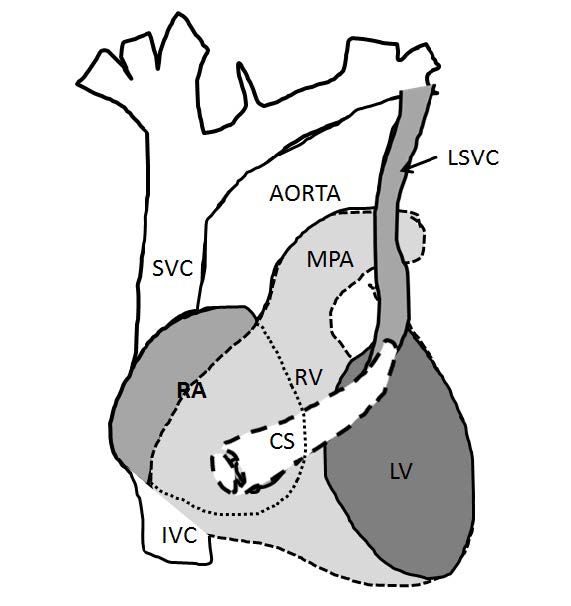
Figure 7.3: Demonstrating diagram of the persistent left superior vena cava (PLSVC) that is emptying into the coronary sinus. The coronary sinus is located at the posterior surface of the heart amd is empting directly into the RA. MPA=main pulmonary artery vessel, CS=coronary sinus.
Congenital pulmonary artery atresia (also known as pulmonary atresia) is a congenital cardiovascular anomaly that is caused by a complete disruption between the right ventricular outflow tract and the main pulmonary artery. The incidence of pulmonary artery atresia is about 0.01% of live births. Pulmonary artery atresia may occur with or without VSD or with complex cardiac malformation. Pulmonary artery atresia with VSD accounts for 2.5-3.4% of all congenital heart disease and occurs in males more than females.21 To approach pulmonary artery atresia on the imaging, the key characters that must be shown are the disruption of pulmonary artery and the RVOT and may include the VSD and also no forming of the pulmonic valve. The atresia of the pulmonary artery structure and VSD will be detected on the axial plane (gradient echo CINE MRI images on the axial plane is recommended) and the VSD is well confirmed on the four chamber view. The character of pulmonary arteries in case of pulmonary atresia looks like a seagull (and is called seagull appearance). The left and right pulmonary arteries represent the left and right wings of the seagull and the head and the body of the seagull are represented by the very small main pulmonary artery (see Figure 8.2). In addition, in patients with pulmonary atresia the pulmonary circulation may be supplied by a patent ductus arteriosus (PDA), systemic to pulmonary collaterals or plexuses of bronchial and pleural arteries hence MRA (angiography) with contrast gadolinium injection has a role to identify these supplied arteries.22 This case is a good case in practicing how to operate the imaging tool to detect absent organs and complex cardiac malformation on imaging(see Figure 8.1-8.4).
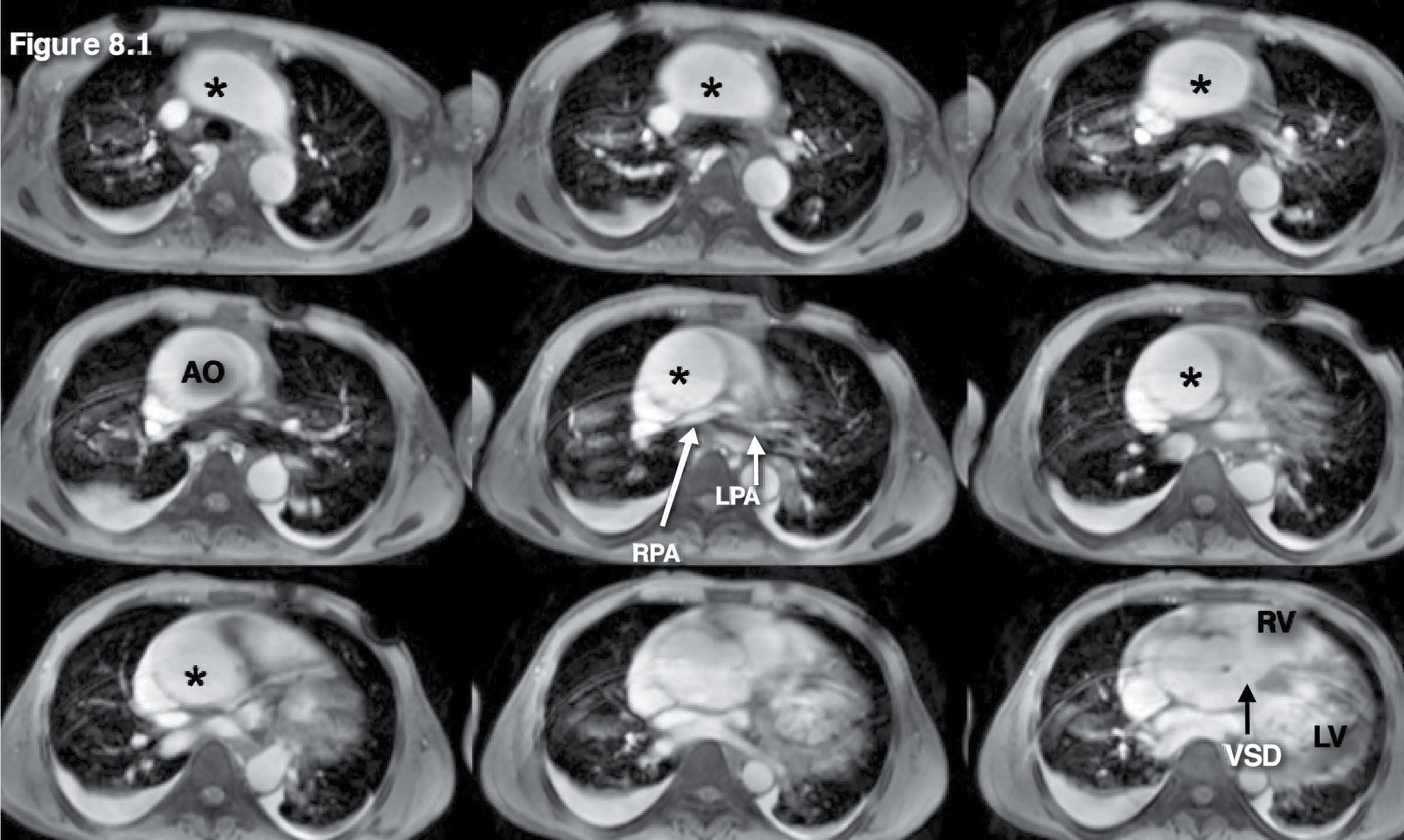
Figure 8.1: Pulmonary artery atresia case: The gradient echo CINE MRI images on the axial plane are used as scout images. They show prominent aorta and membranous VSD with absence of the main pulmonary artery and the very small right pulmonary artery (RPA) and left pulmonary artery (LPA). Pulmonary atresia with membranous VSD is suggested.* = aorta
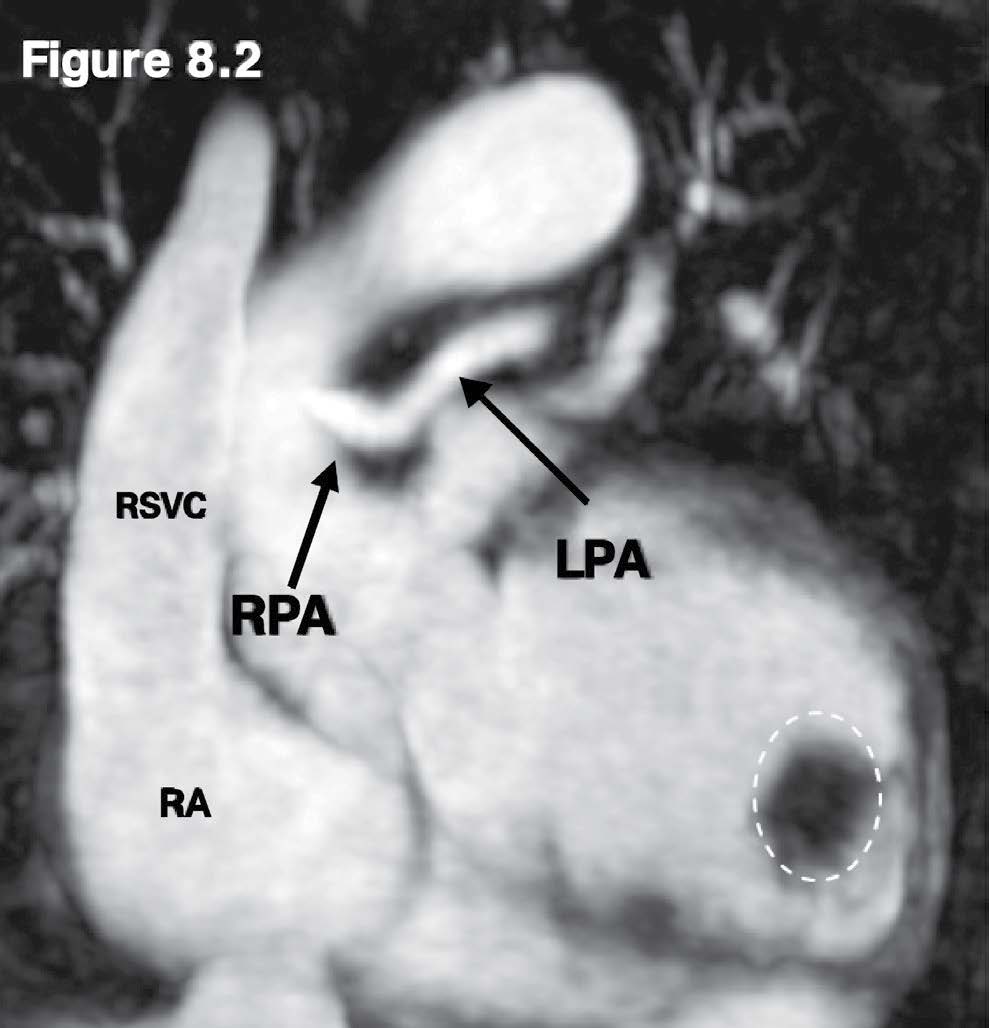
Figure 8.2: Pulmonary artery atresia case: The MRA with gadolinium contrast injection reveals the small right and left pulmonary artery (RPA/LPA) with the absence of the main pulmonary artery (MPA). The sizeable LV apical clot (white dashed circle) is observed.
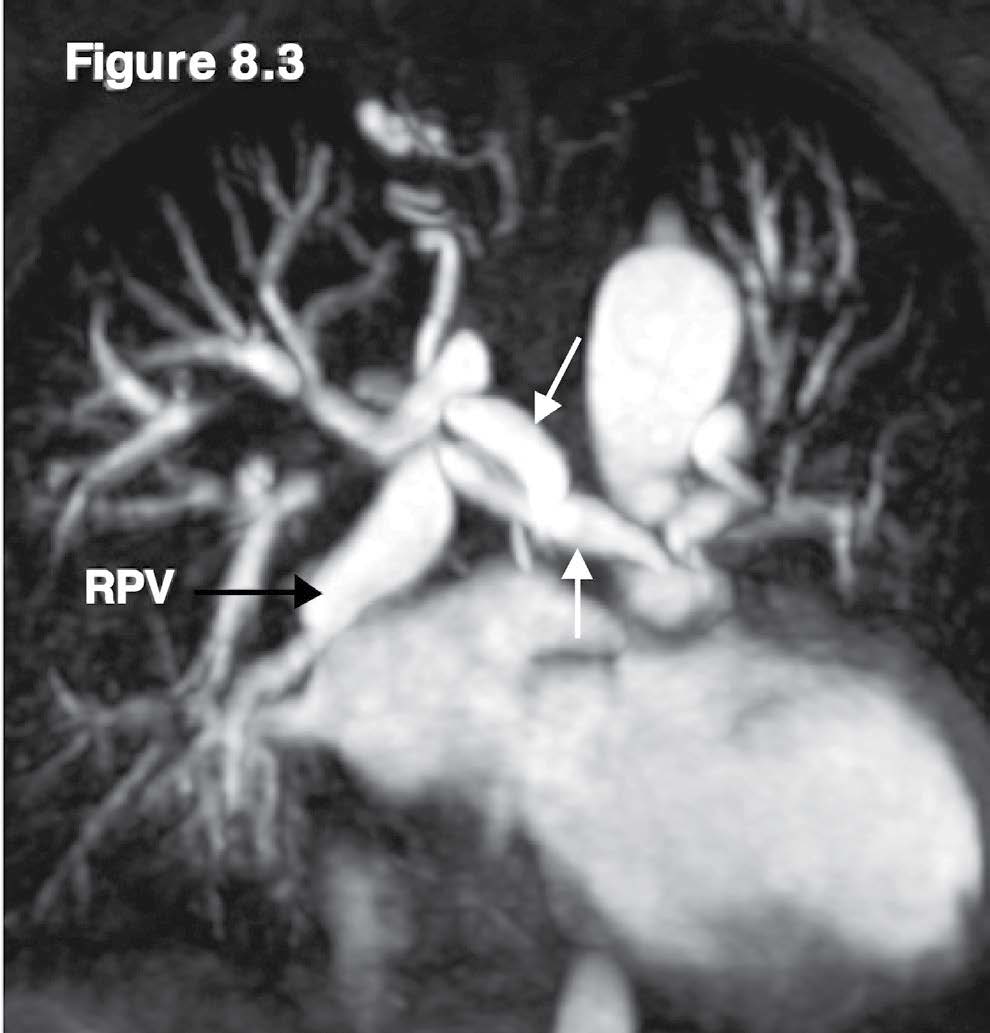
Figure 8.3: Pulmonary artery atresia case: The MRA with adolinium contrast injection reveals the enlarged right pulmonary veins and the collaterals from the aorta (white arrow).
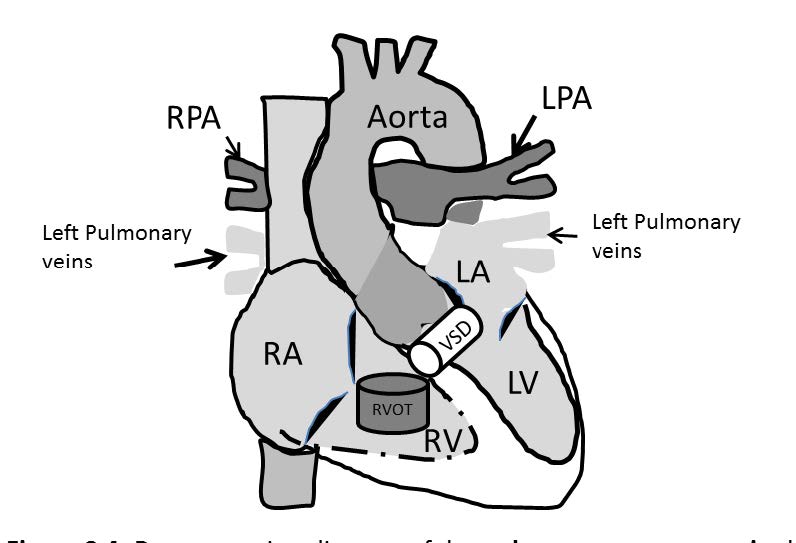
Figure 8.4: Demonstrating diagram of the pulmonary artery atresia shows a complete disruption between the right ventricular outflow tract (RVOT) and the main pulmonary artery (pulmonic valve is not formed) and the ventricular septal defect (VSD). RPA and LPA=right and left pulmonary artery respectively.
PAPVR is the abbreviation of the Partial Anomalous Pulmonary Venous Return. Scimitar PAPVR is named for the PAPVR that is produced by an anomalous pulmonary venous return that drains blood from one or all pulmonary veins of the right lung into the inferior vena cava (IVC).23 Scimitar PAPVR is not the same as scimitar syndrome. Scimitar PAPVR is the one pathologic component of the Scimitar syndrome that is composed of the following characteristics: dextro-cardia, hypoplasia of the right pulmonary artery and the right lung and anomalous arterial supply of the right lower lobe from the abdominal aorta. The draining course of the right pulmonary veins into the IVC of Scimitar PAPVR forms a Turkish sword or Scimitar –like curve shadow on chest x-ray along the right cardiac border. In addition the Scimitar sign of PAPVR is also produced by the draining course of the right pulmonary veins that terminate at the portal vein, hepatic vein or the right atrium[24]. Normally, no vascular branches join with the IVC region above the right thoracic diaphragm. By this key information, the emptying port of the IVC is the main point to depict the anomalous connection with the right pulmonary veins. A scout scan with gradient echo CINE MRI on the axial plane with whole heart coverage is still useful. The IVC at the region above the right sided thoracic diaphragm always performs the complete round curve through the emptying port on the MRI images on the axial plane. PAPVR with a scimitar sign is well confirmed by using MRA with gadolinium contrast injection of the great veins in both the first pass and delayed phase. In addition, the associate syndrome is able to be assessed on the gradient echo CINE MRI on the axial plane images. The MRA with gadolinium contrast injection also displays the anomalous arterial supply of the right lower lobe from the abdominal aorta. [see Figure 9.1-9.4]
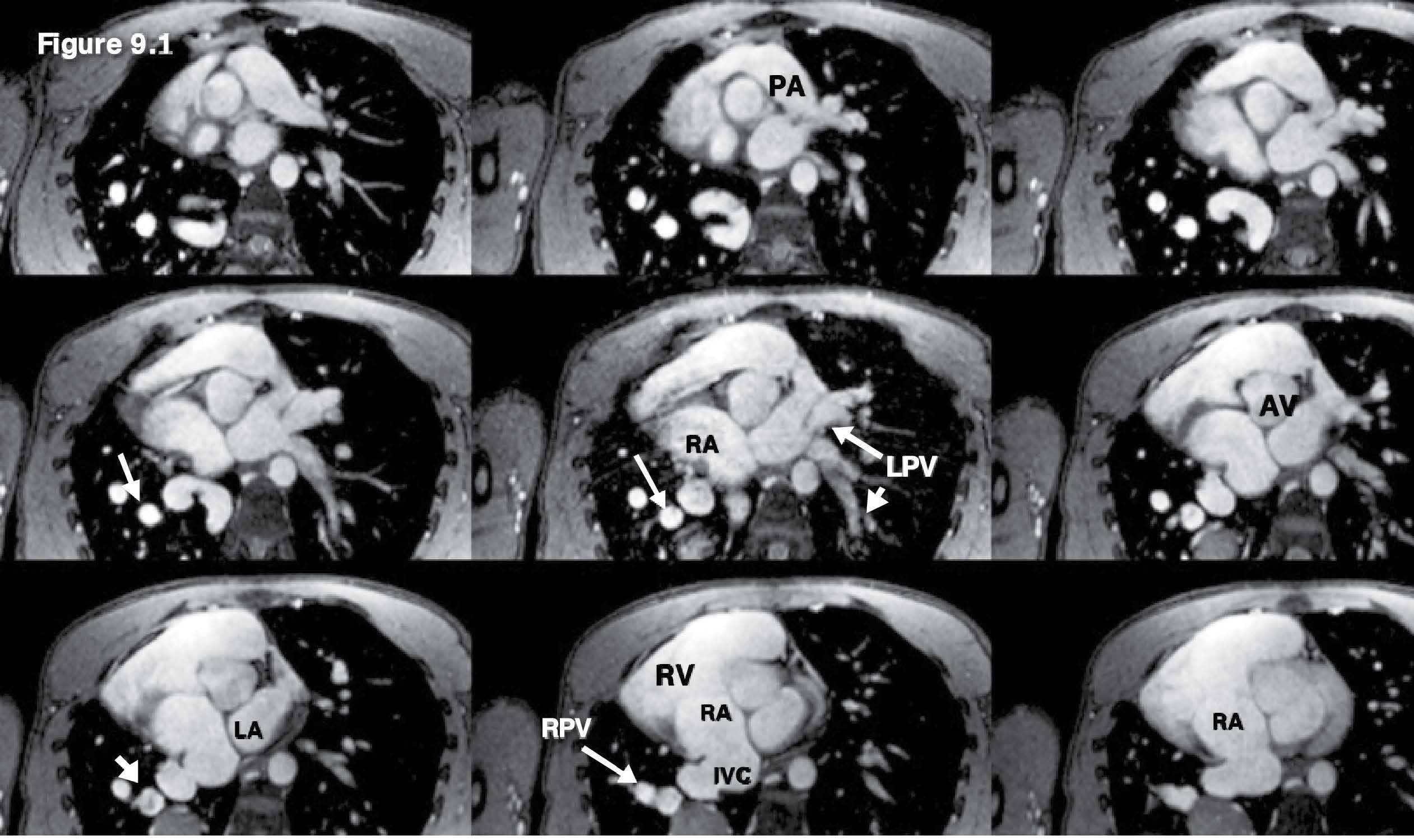
Figure 9.1: Scimitar PAPVR case: The multi-slice images with whole heart and great vessels coverage of the gradient echo CINE MRI on axial plane are used as scout images. The images reveal scimitar syndrome (dextro-cardia) hypoplasia of the right pulmonary artery and the right lung and anomalous arterial supply of the right lower lobe from the abdominal aorta (the right sided PVs emptying into the IVC at the right atrial–IVC junction above the diaphragm). The vessel branch joining with the IVC at the region of emptying port into the RA is a suspicious clue for Scimitar PAPVR.
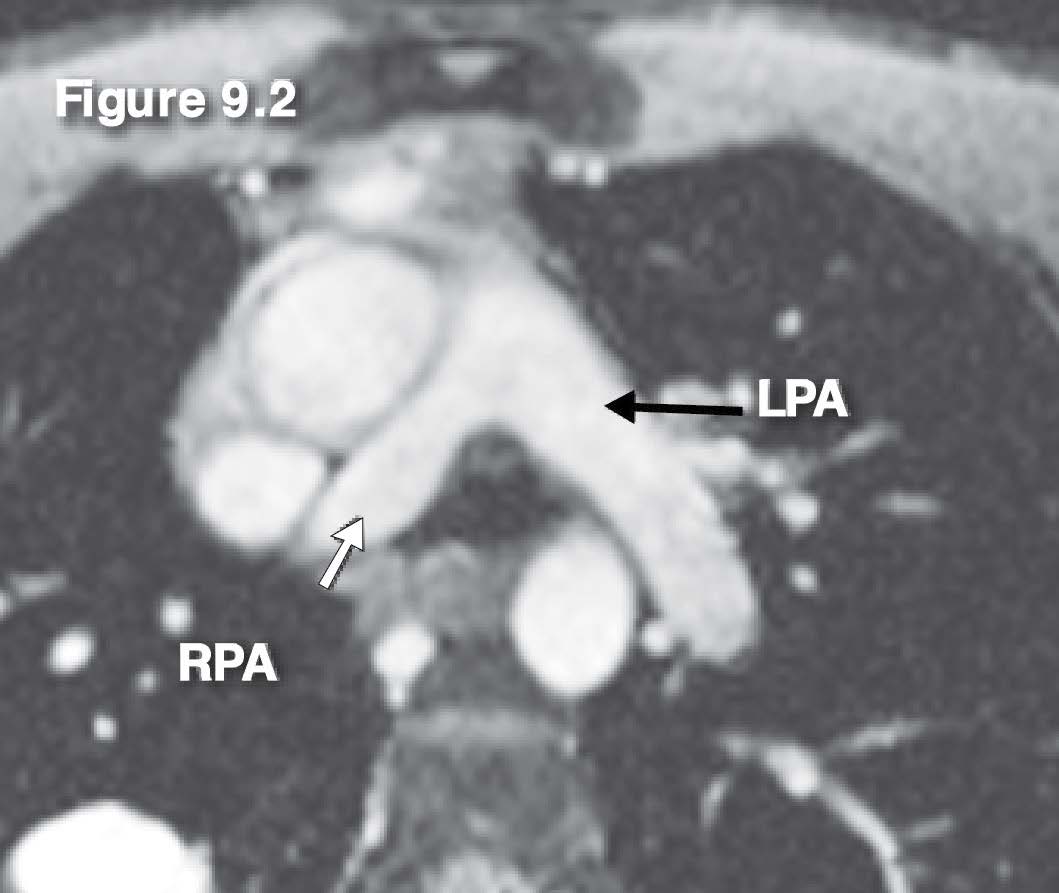
Figure 9.2: Scimitar PAPVR case: The images of the gradient echo CINE MRI on the axial plane show a slightly smaller RPA.
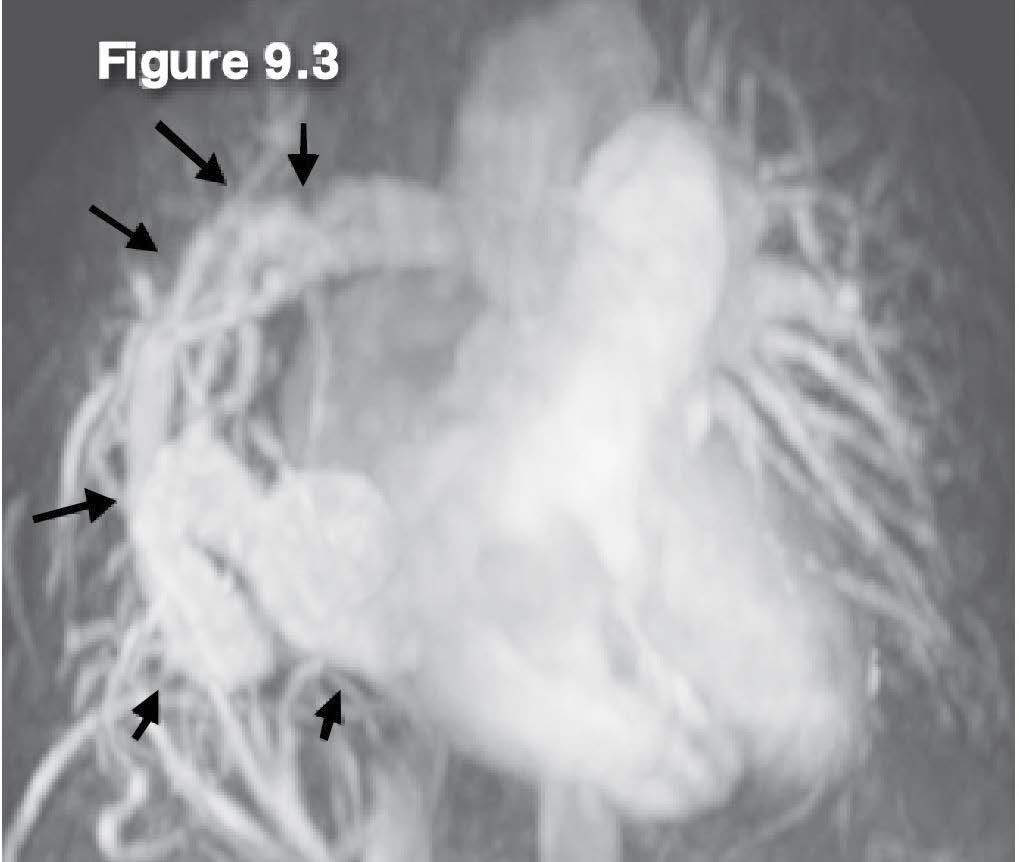
Figure 9.3: Scimitar PAPVR case: The MRA with gadolinium contrast injection reveals Scimitar PAPVR of the mid right pulmonary veins (arrow sign) drainage into the IVC. AV= aortic valve, IVC= inferior vena cava, RA= right atrium, RV =right ventricle, RPV= right pulmonary vein, LPV = left pulmonary vein.
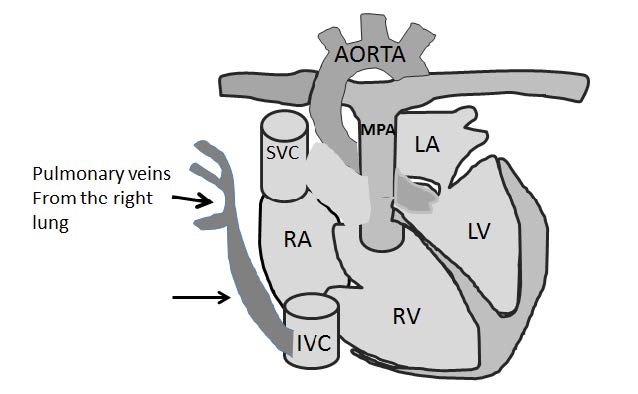
Figure 9.4: Demonstrating diagram of the Scimitar PAPVR that is caused by the blood drainage from the right pulmonary veins into the IVC which produces a Turkish sword (Scimitar)-liked curve shadow on chest x-ray along the right cardiac border (black arrow sign).
Truncus arteriosus is a rare complex congenital heart disease that is caused by the failure of complete separation between the aorta and the pulmonary artery during the fetus development. Truncus arteriosus is composed of the single common arterial trunk as a stem and forms the branches by the pulmonary arteries. The pulmonary artery branches originate from the aorta trunk distal to the coronary artery origin.25 The common trunk always stands astride the inter-ventricular septum that causes the ventricular outlet defect. To demonstrate the abnormalities of the suspected Truncus case, we need to identify which particular type of pulmonary artery is arising from the aortic common trunk as these are classified as 4 primary types.26 The associate anomalies of Truncus that may be found are the dysplastic and an exceeding number of the leaflets with significant regurgitation of the truncal valve, single coronary artery, right sided aortic arch, persistent left superior vena cava, aberrant subclavian artery, and atrial septal defect and a few rare associated anomalies such as complete atrio-ventricular septal defect, double aortic arch, and various forms of functionally univentricular heart. The key pathologic characteristics that will be seen on the imaging are the overriding of the root with one semilunar valve of the common arterial trunk that receives blood from the LVOT and the RVOT and gives branches to the left and right lungs. The scout diagnostic gradient echo CINE image on the axial view can display this key pathology including other anomalies such as PLSVC and sub- valvular VSD. The gradient echo CINE MRI on the sagittal view can be used to classify the type of Truncus. MRA with gadolinium injection of the great vessels is a very useful technique to characterize the congenital malformation of the vessels such as truncus with its pulmonary artery branches, the PLSVC and PDA of their whole running course (see Figure 10.1-10.4).
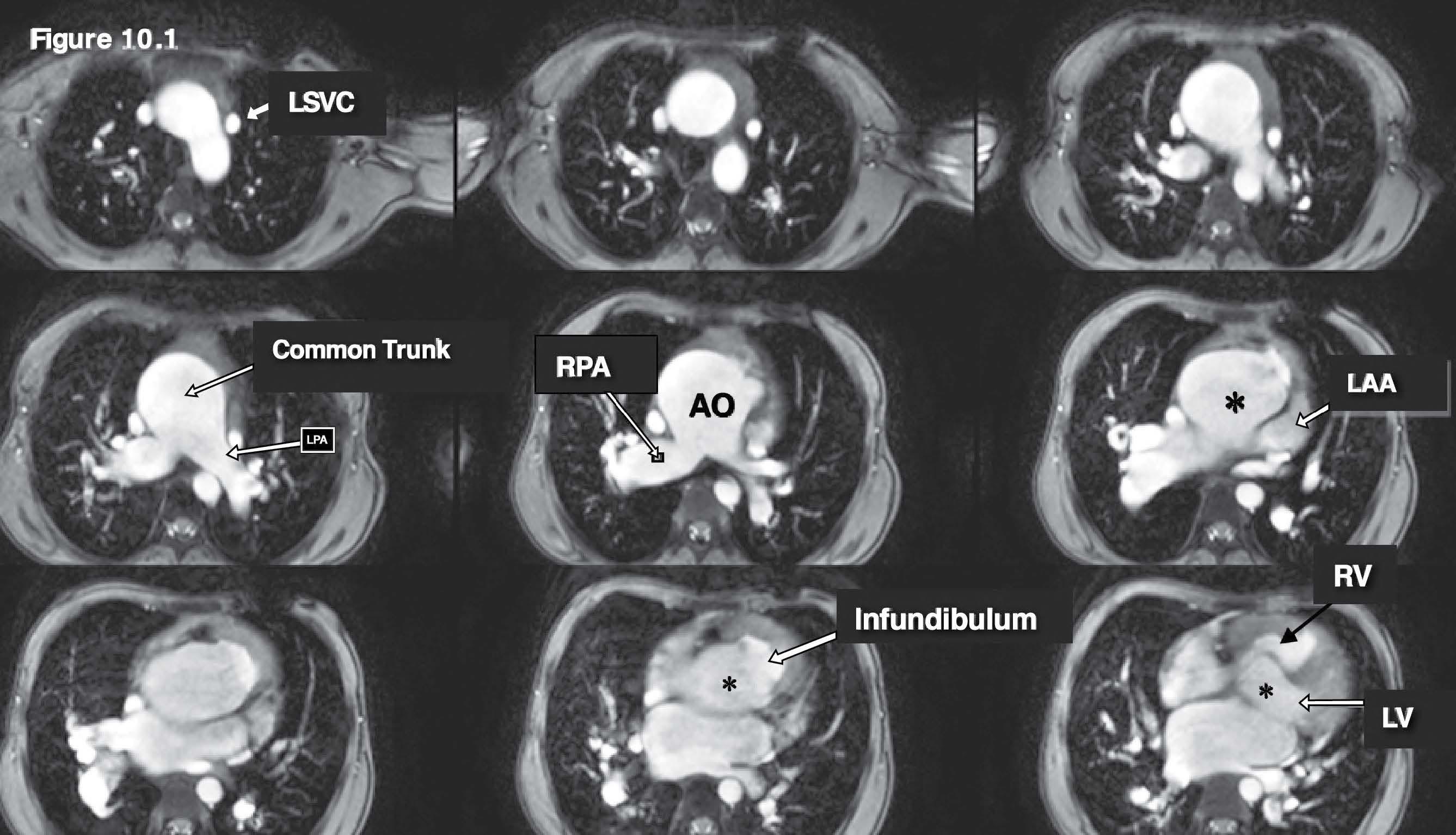
Figure 10.1: Truncus case: The gradient echo CINE MRI on the axial plane images are used as scout images and they reveal a huge common arterial trunk (aorta) and its pulmonary artery branches. The overriding of the common arterial trunk (LVOT and RVOT pump blood flow to the common trunk) including the persistent left SVC (LSVC) which is emptying into the coronary sinus. AO= aorta, RPA= right pulmonary artery , LV = left ventricle , RV= right ventricle.
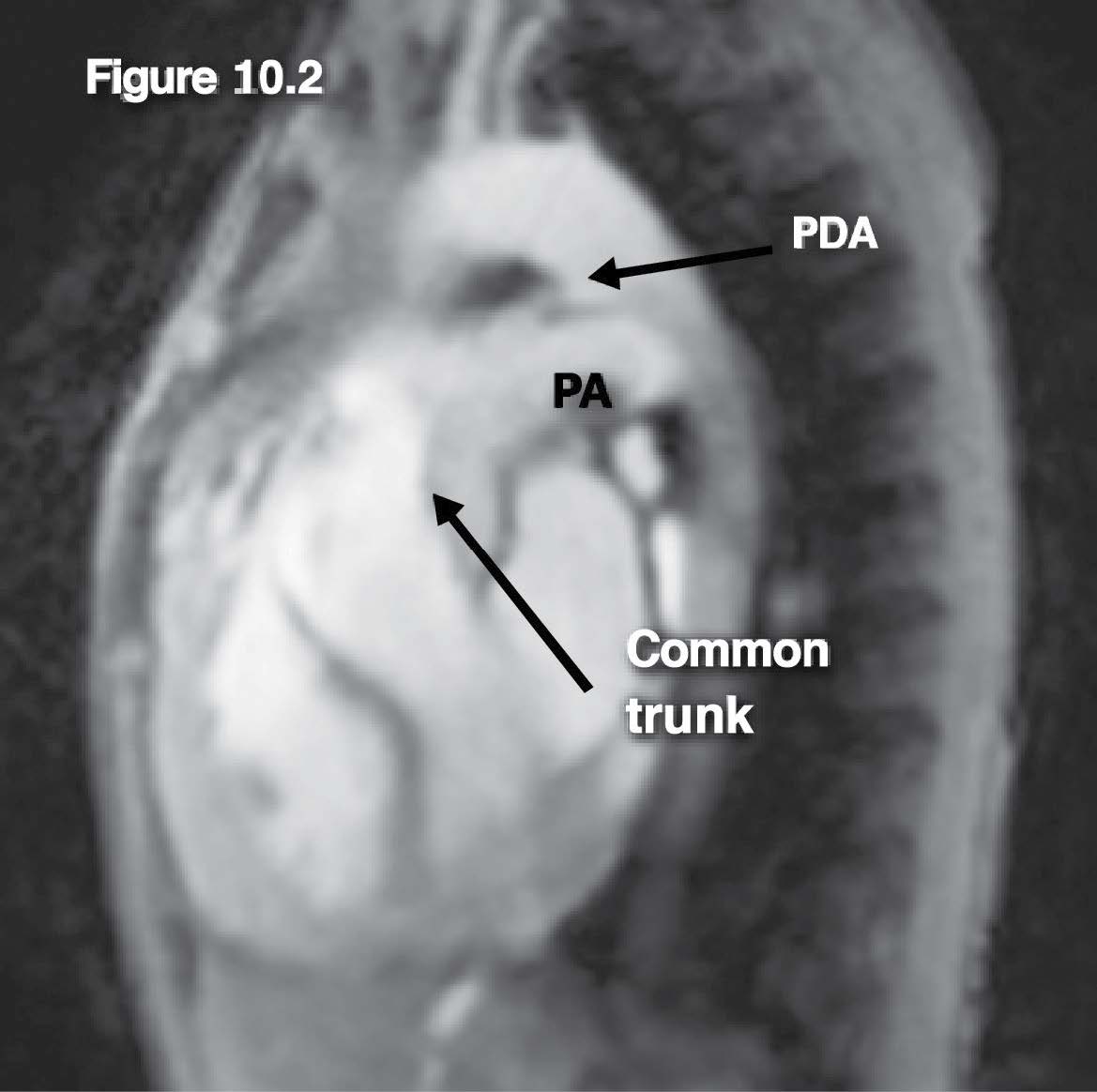
Figure 10.2: Truncus case: The gradient echo CINE MRI image on sagittal view reveals the common arterial (aorta) trunk structure with its pulmonary artery branch and the PDA (patent ductus arteriosus) that forms the connection between the proximal thoracic descending aorta at the ductal region and the left pulmonary artery branch.
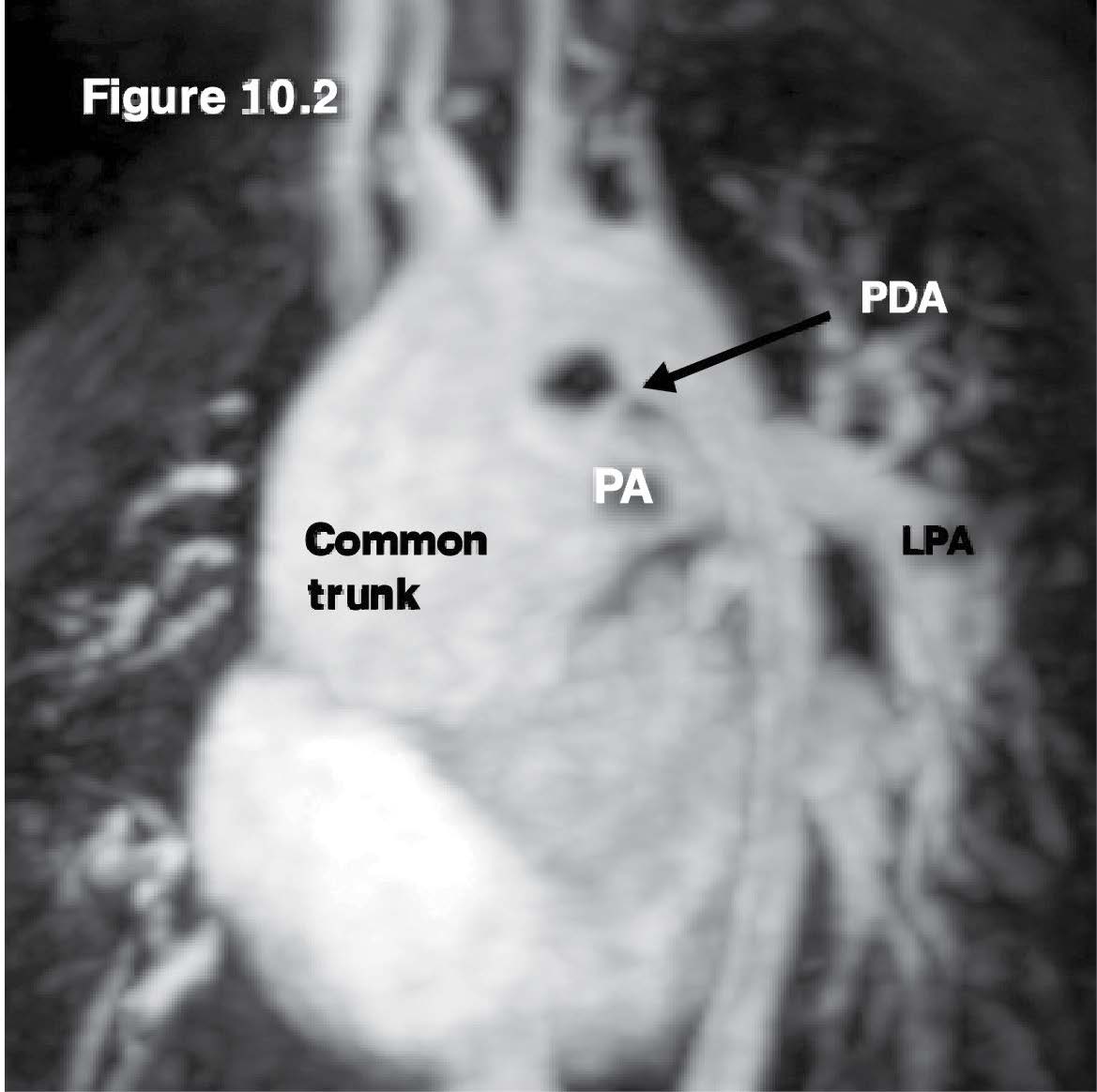
Figure 10.3: Truncus case: MRA with gadolinium contrast injection image demonstrates the common arterial (aorta) trunk structure with its pulmonary artery branch and the PDA that forms the connection between the proximal thoracic descending aorta at the ductal region and the left pulmonary artery branch. PA= pulmonary artery
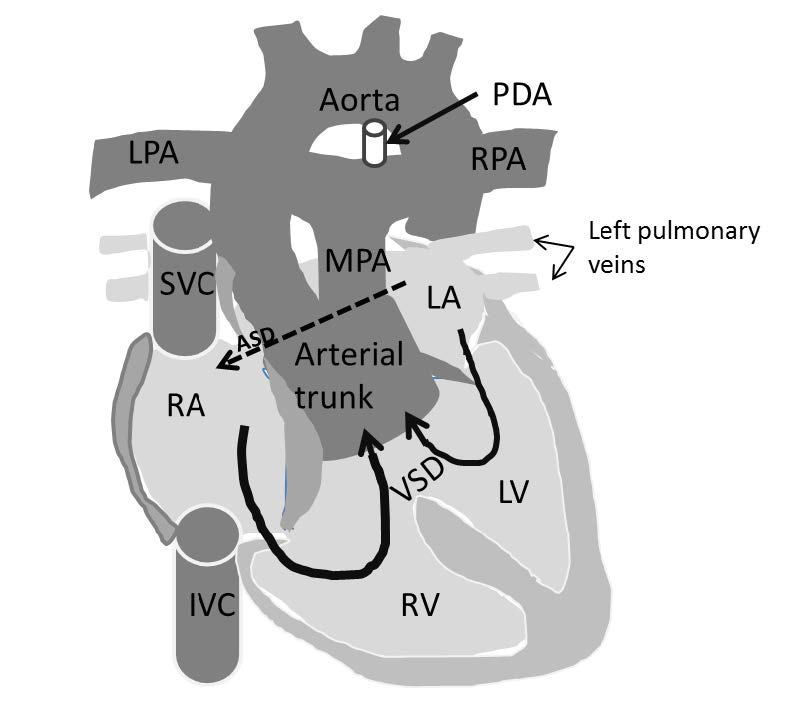
Figure 10.4: Demonstrating diagram of the Truncus arteriosus that is composed of a single common arterial trunk as a stem (arising from the normal ventricle) and forms the branches by the arising of the pulmonary arteries. The associated disease such as PDA (patent ductus arteriosus) and sub-valvular VSD and ASD (atrial septal defect) are also shown.
Through ten cases of rare congenital heart disease demonstrated on MRI, the reader can recognize that the main clue for CHD diagnosis is hiding in the definition of disease. The aim of this article is to share the experiences in image operating strategies to obtain the key diagnostic images that display the main pathologic structure on MRI quickly and correctly. The successful transformation of the pathology of disease to the correct diagnostic images comes from the comprehensive knowledge of the cardiovascular anatomy and structural character of each cardiovascular organ in each image technique and plane. To study about the disease of interest and find out the diag-nostic clue pathology that is quickly discovered from the explanatory definition leading to the correct protocol planning for all congenital heart diseases diagnosis and can be used for protocol preparation for diagnosis of unseen assigned cases. This article shows mainly how to obtain key images to identify the anomaly structures from the explanation of the definition of disease. At least, the correct diagnosis is obtained and the associate disease identification can be done after by the same process. In this article we recommend the gradient echo CINE MRI on the axial view with coverage of the whole heart and thoracic great vessels in all directions (anterior-posterior, superior–inferior and right-left direction) to be used as a scout scan technique to identify the defect anatomy. The great vessels such as the aorta, the pulmonary artery, the SVC, IVC are not distorted by this imaging technique, and this makes it easier for the operator to capture the presence or absence of the abnormal structure. The multi-slice images of gradient echo CINE MRI on the four-chamber view with whole heart coverage is recommended for displaying the defect of the inter-atrial and inter ventricular septum. MRA angiography with contrast injection is the best technique to demonstrate the sizeable vessel anomaly from its origination, the running course and the termination site. After the diagnostic imaging has been done, the interpretation of the imaging result step by step following the sequential segmental analysis principle is recommended. This principle is very helpful for completing the diagnosis of complex and complicated congenital heart disease.2
I am extremely thankful to all staff of the cardiac imaging team of the Bangkok Heart Hospital for their kind help throughout my work and all my colleagues at the alliance hospital for their trust. I would like to deeply thank Bangkok Heart Hospital (Bangkok Hospital) for their generous support.