Migraine is a common recurrent neurological disorder with a high impact and is debilitating. Approximately 14.7% of the global population has migraine (an estimated 6% in men and up to 18% in women).1 A recent report ranked migraine headache as the third most common disease in the world (behind dental caries and tension type headache).2 On the basis of migraine attack disability alone, migraine was ranked seventh highest among specific causes of disability globally (responsible for 2.9% of all years of life lost to disability).1,2 Recent data from US public health surveillance studies showed that headache was the fifth leading cause of emergency department (ED) visits in the United States, estimated to account for over 4 million visits annually (1.2% of outpatient visits). The burden of headache was highest in females aged 18-44, where the 3-month prevalence of migraine or severe headache was 26.1% and head pain was the third leading cause of ED visits.3 Most migraineurs who visit ED for treatment generally have severe and/or prolonged symptoms concomitant with nausea/ vomiting, and usual acute medications are not effective. Over half of the patients use simple analgesics to treat their headache attack but often to no effect.4 Migraine-specific medications (triptans or ergotamine) have been used only in a few patients.5 The goal of ED therapy is to deliver medicines with rapid, complete relief of pain and associated migraine symptoms, restore functional ability, with minimum adverse effects and without recurrence of headache after ED discharge.6 Intravenous medications that are used specifically for rescue treatment in migraine included dopamine antagonists, NSAIDs, opioids, magnesium, valproate, and corticosteroids. This paper consists of 2 parts; part 1 reviews the role of dopamine antagonists, NSAIDs, and opioids that are available in Thailand for migraine treatment in ED.
A detailed history and physical examination can help confirm the diagnosis and rule out life-threatening causes of secondary headaches such as subarachnoid hemorrhage, arterial dissection, meningitis, temporal arteritis, cerebrospinal fluid outflow obstruc- tion, and elevated or low intracranial pressure. The clinical charac- teristics of secondary cause of headache can be memorized from the mnemonic “SNOOP4”:
Systemic symptoms (fever, weight loss) or Systemic disease (malignancy)
Neurologic symptoms or signs
Onset sudden (acute or thunderclap headache)
Onset after age 50 years
Previous headache history (new or different)
Progressive
Precipitation by Valsalva (cough, bend)
Postural
If one or more of the SNOOP4 features are present, the diagnostic tests including neuroimaging (computed tomography or magnetic resonance imaging), lumbar puncture, and blood tests may be considered.7
Migraine is diagnosed by clinical criteria provided by the International Classification of Headache Disorders 3rd Edition beta (ICHD-III beta; Table 1).8 Migraine head- ache is characterized by unilateral location, pulsating quality, moderate to severe intensity, aggravated by routine physical activity, and accompanied by photophobia (sensitivity to light) and phonophobia (sensitivity to sound) or nausea and/or vomiting.
The accurate diagnosis of headache can be troublesome at the ED due to the severity of the migraine attack, the associated symptoms especially nausea and/or vomiting that might require emergency treatment, and also because of the complicated ICHD-III beta criteria. A brief self- administered migraine screener (ID-Migraine), consisting of questions on disability, nausea, and photophobia, is a valid and reliable screening instrument for migraine headaches in the primary care setting. The test outcome is positive when the answer is “yes” for at least two out of the three questions.9 (Table 2)
The study role of ID-Migraine in emergency departments showed that the ID-Migraine exhibited a high sensitivity of 0.94 and high specificity of 0.83 with high positive predictive value (PPV) of 0.99 in primary headache.10 A negative ID Migraine score, less than 2 positive responses, reduces the post test probability of migraine from 59% to 23%.11
Management for acute migraine in the emergency room
Intravenous fluid
The patient should be assessed for volume depletion because nausea occurs in more than 90% and vomiting occurs in approximately 70% of migraineurs. In those who experienced nausea or vomiting, 30.5-42.2% indicated that it interfered with their ability to take their oral migraine medication.12,13 Intravenous fluid replacement is useful for rehydration and in avoiding postural hypotension associated with dopamine antagonist treatment and provides some degree of renal protection if non-steroidal anti-inflammatory drugs (NSIADs) are used.14,15 A quiet, darkened and comfortable area in ED can provide additional benefits to these patients.
Dopamine antagonists (Neuroleptics)
Dopamine plays an important role in migraine patho- genesis probably by acting on central pain control pathways and on cranial blood vessels.16 Recent genetic studies show evidence of genetic polymorphism of the dopaminergic and dopamine transporter genes.17,18 The dopamine metabolite is increased in cerebrospinal fluid (CSF) during the migraine attack and the levels correlate with the severity of pain.19 Dopamine antagonists are very useful in rescue treatment of migraine and appear to be equivalent to the migraine specific medications (sumatriptan and dihydro- ergotamine) in migraine pain relief.20 There are 2 major subclasses of neuroleptics, the phenothiazines group (e.g., prochlorperazine, chlorpromazine, promethazine, and methotrimeprazine) and the butyrophenones group (droperidol and haloperidol). Metoclopramide is in its own third class. The phenothiazines, chlorpromazine and prochlorperazine, were commonly used in ED. Thirteen trials showed that phenothiazines were markedly more effective than placebo for headache relief (OR 15.02; 95% CI, 7.57-29.82) and clinical success (OR 8.92; 95% CI, 4.08-19.51). The phenothiazines were more effective than metoclopramide (OR 2.25; 95% CI, 1.29-3.92) for clinical success, but not different for headache relief. The clinical success rate of phenothiazines was 78% (95% CI, 74-82).21
Neuroleptics act on the postsynaptic dopamine D2 receptor in the hypothalamus, limbic system, periaqueductal grey, and basal ganglia. Neuroleptics also have anticholinergic, antiadrenergic, anti-serotonergic and antihistaminergic effects. The most common side effects of neuroleptics are sedation and drowsiness due to blockage of muscarinic cholinergic and histamine receptors. Extrapyramidal side effects dystonia and akathisia are commonly seen in parenteral form and more often with prochlorperazine. Dystonia and akathisia can be prevented by premedication with anticholinergic agents (e.g., benztropine, diphen- hydramine, or trihexyphenidyl). Due to a-adrenergic antagonist effects, postural hypotension can occur but is infrequently reported in phenothiazine. Neuroleptic malignant syndrome, which is characterized by fever, rigidity, confusion, and autonomic function instability, is a rare side effect. Droperidol and haloperidol can cause QT interval prolongation that increases risk of fatal ventricular arrhythmias and cardiac arrest.21
- Chlorpromazine
Several randomized controlled trials and head-to-head trials supported the efficacy of chlorpromazine in acute migraine.20 Pool clinical analysis showed the success rate of acute migraine treatment is 81% for chlorpromazine (95% CI, 75-86%).23 In a randomized controlled trial in an emergency room setting, chlorpromazine at dose 0.1 mg/kg intravenous (IV) showed a significantly reduced pain score compared with placebo in 128 migraineurs. Pain free at 1 hour for migraine with aura (66.7% in chlorpromazine group vs. 6.7% in placebo; p < 0.01) and migraine without aura (63.2% vs. 10%; p < 0.01).24 In comparison trials, pain reduction (VAS) was greater for chlorpromazine 0.1mg/kg IV (up to 3 doses) than meperidine 0.4 mg/kg IV plus diphenhydramine 25mg (VAS -70.6 vs. -44.5; p < 0.05).25 Chlorpromazine 12.5 mg IV (could be repeated up to 37.5mg) was compared with lidocaine 50mg IV (could be repeated up to 150 mg) and with dihydroergotamine (DHE) 1 mg IV (could be repeated once). Pain reduction (11 PPS) was greater with chlorpromazine than with lidocaine or DHE (chlor- promazine -79.5% vs. lidocaine -50% vs. DHE -36.7%; p < 0.05).26 Chlorpromazine 12.5 mg IV (could be repeated up to 37.5mg) was compared with sumatriptan SQ 6mg. At 2 hours, there was no difference in pain reduction (VAS) (chlorpromazine -54.3 mm vs. sumatriptan -63.3 mm).27
Table 1: The International Classification of Headache Disorders, 3rd edition (beta version) for migraine without aura and migraine with aura.
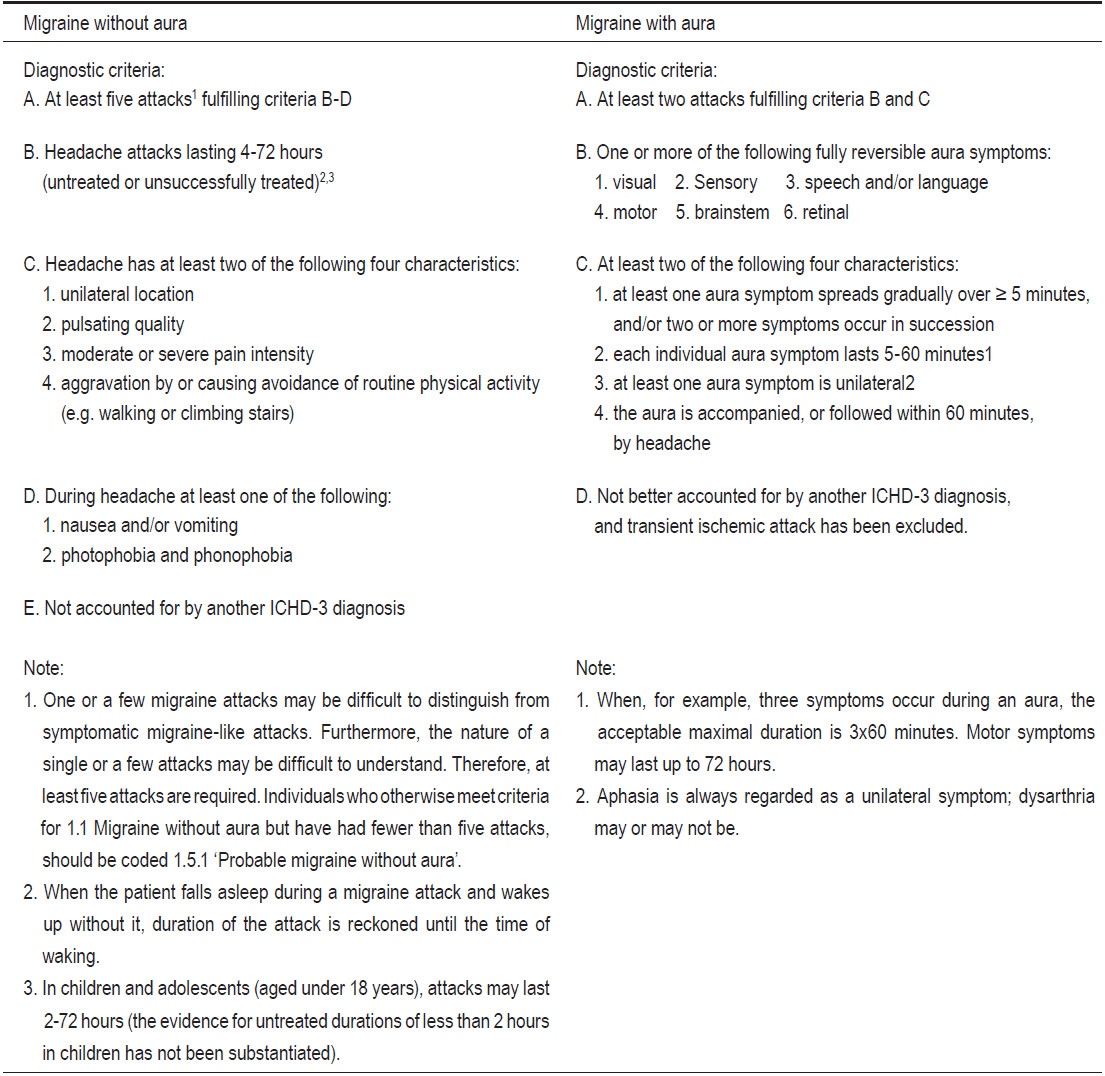
Table 2: The ID-Migraine.

- Metoclopramide
Metoclopramide is widely available, inexpensive, and commonly used for the treatment of nausea, vomiting, and gastroparesis. In addition to dopamine antagonists, metoclopramide also has serotonin antagonist effect. Common side effects include fluid retention (caution in congestive heart failure and liver disease), lower seizure threshold, mild sedation and extrapyramidal side effects. Meta-analysis from 13 randomized controlled trials for parenteral metoclopramide in acute migraine showed metoclopramide is an effective treatment for migraine headache in adults and should be considered as a primary agent in the treatment of acute migraine in emergency departments.28 Metoclopramide 10mg IV showed superior to placebo in three studies for all outcomes related to pain and nausea. Pooled data from three studies showed that metoclopramide more often leads to significant reduction in headache pain (OR 2.84; 95% CI, 1.05-7.68).28 Meto- clopramide was more effective than placebo in reducing nausea in four studies.29,31,32
Three studies compared metoclopramide with other neuroleptics (chlorpromazine and prochlorperazine).30,33,34 These studies suggested that metoclopramide was less effective in relieving pain and nausea than phenothiazine. Pooled results from all three studies showed that patients who received metoclopramide were more likely to require rescue drugs (OR 2.0; 95% CI, 1.04-4.17).28 In comparison to non-emetics, metoclopramide 10mg IV was similar in pain reduction to metoclopramide plus ibuprofen 600 mg PO (VAS -75 vs. -50) but VAS reduction was larger than ibuprofen alone or placebo. Patients in the metoclo- pramide group were significantly less likely to require rescue drugs.31 Metoclopramide 10mg IV was compared with magnesium 2g IV and with placebo. Pain reduction was similar in three groups but in metoclopramide and magnesium groups the requirement of rescue medications was less than in placebo (38% and 44% vs. 65% in placebo; p = 0.04).34 Metoclopramide 20mg IV plus diphenhydramine 25mg IV (dose up to 4 times) was found to be superior to sumatriptan 6mg SQ as a percentage of pain free response at 2 hours (59% vs. 35%; p = 0.04) but not for 1 hour (p = 0.22) and 24 hours (p = 0.23).36
In a dose-finding randomized double-blind clinical trial of 356 patients, intravenous metoclopramide 10mg had similar efficacy as metoclopramide 20 or 40mg in pain reduction (11PPS) at 1 hour (-4.7 vs. -4.9 vs. -5.3; p = 0.19). Sustained pain free rate was low in all doses (16% vs. 20% vs. 21%). The most common adverse event was drowsiness (69% at 1 hour), which impaired function in 17%. Akathisia developed in 9% and dizziness in 8% with similar rates across doses.37
- Haloperidol
Haloperidol is a butyrophenone derivative acting mainly by blocking D2 dopamine receptor with some antagonist with D1 dopamine, 5-HT2 serotonin, H1 histamine, and a2 adrenergic receptors in the brain. Haloperidol 5 mg in 500 mL normal saline (NSS) IV was compared with placebo NSS 500 mL IV. Pain reduction (VAS) was greater with haloperidol (from 7.7 to 2.3; p < 0.0001) compared to placebo (from 7.2 to 6.3; p < 0.01). Eighty percent who received haloperidol felt marked relief from pain, whereas only 15% responded to placebo (p < 0.0001). The common side effects were motor agitation (akathisia) in 53% and drowsiness in 53%. Sixteen percent of the patients considered the side effects intolerable and were unwilling to be treated with haloperidol in the future.38 Due to black box warnings for prolonged QTc, together with high incidence of sedation and akathisia, haloperidol should be reserved for use only when other rescue medications failed to relieve headache. Electrocardiogram (ECG) should be done before and after treatment. Pretreatment with IV fluid and an anticholinergic (benztropine, trihexyphenidyl, diphenhydramine or benzodiazepine) are recommended.20
Nonsteroidal anti-inflammatory drugs (NSIADs)
NSAIDs inhibit both cyclooxygenase-1 (COX1) and cyclooxygenase-2 (COX2), the neurogenic inflammatory cascade and thereby prostaglandin synthesis, and platelet aggregation associated with the release of vasoactive agents that are involved in initiation and prolongation of migraine.39
- Ketorolac
Ketorolac is a NSAID with strong analgesic activity. Ketorolac attenuates moderate to severe pain especially in patients with migraine headache and is usually as effective as an opioid. The analgesic effect of ketorolac tends to be slower in onset than that of morphine or meperidine but persists longer.40 Most adverse events involve the gastro- intestinal tract (GI), ranging from mild upset to ulceration and hemorrhage. Overall, parenteral ketorolac is associated with only a slightly increased risk of GI or operative site bleeding compared to opioids (OR 1.30 and 1.02, respec- tively). The risk of bleeding is strongly linked to increased age, high dosage and treatment for more than 5 days.41 All NSAIDs have the potential to cause nephropathies, but these occur more frequently in patients with hypovolemia or patients with hemodynamic compromise.42
The total daily dosage of parenteral ketorolac should not exceed 60mg in patients aged c 65 years or those with renal impairment or body weight < 50kg. Ketorolac is contraindicated in patients with congestive heart failure, hepatic impairment, hypertension or conditions that may lead to a reduction in blood volume and in those who are hypersensitive to NSAIDs. Ketorolac should not be co-administered with other NSAIDs, probenecid, pento- xifylline or lithium and should be administered cautiously to patients on anticoagulation therapy (including low dose heparin).40 High quality evidence from randomized controlled trial studies (RCTs) shows ketorolac efficacy; it is used in up to 16% of ED visits for migraine.43 The US FDA approved parenteral ketorolac for abortive treatment of migraine. A recent systematic review which included 8 RCTs (321 patients) examined the effectiveness of ketorolac in acute migraine and suggested ketorolac is associated with pain reduction in adult migraine attack patients.44 Ketorolac (30-60mg IM) had similar efficacy to meperidine (50-100 mg IM) in pain score reduction at 60 minutes (weight mean difference (WMD) 0.44; 95% CI, -0.4 to 1.38, p = 0.35). Due to risk of abuse and addiction associated with meperidine, ketorolac is a preferred agent in ED. Furthermore, patients with acute migraine who were treated with narcotic agents showed an increased likelihood to return to the ED within 7 days.45
A single study demonstrated that ketorolac IV 30mg was significantly more effective than nasal sumatriptan 20 mg (WMD = -4.07; 95% CI, -6.02 to -2.12).46 Ketorolac has not been compared with other more effective routes of sumatriptan (subcutaneous and oral). However, most patients have often used triptans as an abortive agent before ED arrival. Two studies compared IV ketorolac with IV phenothiazines (prochlorperazine and chlorprom- azine). The pool analysis found no statistical difference in pain relief (WMD 0.82; 95% CI, -1.33 to 2.98). Hetero- geneity was high, trends may suggest phenothiazines are more effective.47,48
- Parecoxib
Parecoxib is a cyclooxygenase-2 (COX-2) specific inhibitor widely used for acute pain in ED. In animal models, parecoxib show significantly attenuated plasma protein extravasation (PPE) in rat dura mater and reduced expression of c-fos within the ipsilateral trigeminal nucleus caudalis (TNC) that are involved in neurogenic inflammation correlated with migraine pathophysiology.49 Only one open-label pilot study investigated the efficacy of intrave- nous parecoxib, oral fast-dissolving tablet of rizatriptan, and subcutaneous injection of sumatriptan in patients with acute migraine. Using the visual analog scale for pain intensity at baseline before and then after the drug intake at intervals of 20, 30, 60, and 120 minutes, rizatriptan 10mg was more efficacious than parecoxib 40mg and sumatriptan 6mg, and parecoxib was more effective than sumatriptan at 20 and 30 minutes after drug administration.50
Opioids
Opioids have long been used to treat various types of pain include headache. Parenteral opioids are very frequently used in the ED setting, for example for more than 50% of all migraine visits to EDs in US and in Canada.51,52 Opioids can modulate nociceptive input to the spinal trigeminal nucleus (nucleus caudalis) but they have no effect on neurovascular inflammation in migraine pathophysiology.53 A large US population-based study demonstrated that opioid use is common for the acute treatment of migraine. Approximately 30% of the popu- lation reported use of opioids for migraine, 15.9% were currently using opioids. Around 16% of current opioid users were dependent. Opioid use for migraine is associated with more severe headache-related disability, symptomology, comorbidities (depression, anxiety, and cardiovascular disease), and greater health-care resource utilization for headache.54 The major concern about the use of opioids in acute migraine attack regards the possible association with an increased risk of medication overuse headache (MOH) and chronic migraine (CM). Overuse of short-acting opioids may be associated with worsening of migraine, increasing the risk of CM and MOH. Clinical- based and population-based longitudinal studies support an association between CM and opioids use.55 In the American Migraine Prevalence and Prevention (AMPP) study, the risk of CM is higher for opioids use (with an OR = 1.48). Critical level of exposure is around 8 days per month, and the effect is more evident in men (OR = 2.76) than in women (OR = 1.28).56
In addition, opioid-induced hyperalgesia (OIH) can occur with even brief durations of narcotic treatment. Of patients who were exposed to opioids, their pain thresholds declined, and their pain sensitivity, pain location, and pain intensity increased to any stimulus. The phenomenon of OIH can be temporarily overcome with increased doses of opioids, but the basic pathophysiology is different from tolerance.57 Pathophysiology of OIH involves cholecystokinin up-regulation in the rostral ventromedial medulla (RVM), peripheral expression of calcitonin-gene related peptide (CGRP) in primary afferent neurons is up-regulated, there is an increase in dynorphin, pro- inflammatory peptides, activation of NMDA glutamate receptors, and activation of glia cell via the toll-like receptor 4 (TLR4), resulting in inflammation and release of neuroexcitatory substances.58 Opioids are pro-nociceptive, prevent reversal of migraine central sensitization, and interfere with triptan effectiveness.59 Adverse effects of opioids include sedation, respiratory depression, bradycardia and hypotension, seizure, in addition to long term dependence.
- Meperidine
Meperidine is the opioid most frequently used and most studied for headache treatment in ED.60 In a meta- analysis of meperidine of 19 RCT trials with a total 254 patients, meperidine had less efficacy than DHE (OR 0.3; 95% CI, 0.09-0.97) and trended toward less efficacy than antiemetics (OR 0.6; 95% CI, 0.19-1.1) but with a similar efficacy to ketorolac (OR 1.75; 95% CI, 0.84-3.61). Meperi- dine caused more sedation (OR=3.52; 95% CI, 0.87-14.19) and dizziness (OR=8.67; 95% CI, 2.66-28.23) than DHE, less pyramidal side effects than antiemetics, and similar rates of gastrointestinal adverse effects (OR=1.27; 95% CI, 0.31-5.15) and sedation (OR=1.70; 95% CI, 0.23-12.72) to ketorolac.61
A review of 75 studies of rescue therapy for acute migraine found opioids (meperidine, tramadol, and nalbuphine) were superior to placebo in relieving migraine pain, although meperidine combined with promethazine was not. Meperidine 75mg was superior to ketorolac 30 mg IM but was similar to ketorolac 60mg IM even when combined with an antihistamine. Meperidine 75mg IM or 1.5mg/kg IV was similar in pain relief to DHE 0.5mg IV but inferior to DHE 1mg IV.62
- Tramadol
Tramadol hydrogen chloride is an atypical opioid that has relatively weak mu opioid receptor binding properties and also inhibits serotonin and norepinephrine re-uptake. Tramadol is better tolerated than other opioids because of its low impact on the respiratory, cardiac, and gastrointestinal systems at therapeutic doses.63 Tramadol 100mg IV was compared with placebo (NSS) IV. Pain reduction (VAS) at 1 hour was greater in the tramadol group (70.6% vs. 35.3%, p=0.04) but the percent pain free at 1 hour was not different in both groups (29.4% vs. 11.8%, p=0.40). Side effects were not observed at 1 hour.64 In the prospective, randomized, double-blind study, tramadol 100 mg IM was compared with diclofenac sodium 75mg IM; headache relief was the same in both groups (80%).65
Migraine is a common disabling disorder. Migraine attacks with moderate to severe pain and other debilitating associated symptoms such as nausea, vomiting, photo- phobia, and phonophobia that lead to emergency room visits. Detailed history taking, physical examination, and appropriate diagnostic tools should rule out secondary headache. The mnemonic “SNOOP4” is a useful for recognizing secondary causes of headache. ID-migraine, the short 3 item questionnaire, is recommended as a screening diagnosis tool for migraine. Intravenous fluid can be useful in patients with nausea and vomiting, and to prevent postural hypotension in patients who will be administered a dopamine antagonist.
Dopamine antagonists (chlorpromazine and metoclo- pramide) are very effective in rescue migraine treatment. Effective doses of chlorpromazine in migraine ranged from 0.1mg/kg to 37.5mg administered intravenously or via intramuscular injection. The efficacy of chlorpromazine in pain relief was up to 80%. Metoclopramide 10mg IV had an average pain relief of 70%. The most common adverse effects of dopamine antagonists were drowsiness, postural hypotension, and akathisia. Dopamine antag- onists are recommended as the first line treatment for migraine attack in ED.
Ketorolac, the parenteral NSAID, should be considered a second line rescue treatment in ED. The recommended ketorolac dose is 30mg IV or 60mg IM. Average percentages of pain relief of ketorolac are 60% for ketorolac 30mg IV and 37% for ketorolac 60mg IM. Ketorolac and meperidine had similar pain scores at 60 minutes and ketorolac was not different from phenothiazines in pain relief at 60 minutes. Adverse effects were risk of GI bleeding and nephropathies that increased with age, high dosages, and prolonged use for more than 5 days.