Alzheimer’s is the most common form of dementia. According to the Alzheimer’s Association, Alzheimer’s disease accounts for an estimated 60 percent of dementia cases.1 It also reported that about one in nine people aged 65 and older (11%) have Alzheimer’s disease and about one-third of people aged 85 and older (32%) have Alzheimer’s disease. According to the National Center for Health Statistics, Alzheimer’s disease is listed as the sixth-leading cause of death in the United States.2 Early clinical symptoms for Alzheimer’s disease include: difficulty remembering names and recent events, apathy and depression.1 Later symptoms include impaired judgment, disorien- tation, confusion, mood and behavior changes and difficulty in speaking, swallowing and walking. Although Alzheimer’s disease is not curable at present, many researchers believe that future treatments to slow or stop Alzheimer’s disease will be most effective when administered during pre-clinical and mild cognitive impairment (MCI) stages of the disease.
The brain pathological findings of Alzheimer’s disease patients are deposits of protein fragment beta-amyloid (plaques) and twisted strands of the protein tau (tangles) as well as evidence of nerve cell damage and death in the brain.1 The definitive confirmation of Alzheimer’s disease is by postmortem histopathological examination of beta-amyloid deposits in the brain. Therefore, beta-amyloid in the brain is one of the useful biomarkers for differential diagnosis of Alzheimer’s disease. Biomarkers can be used to indicate the earliest signs of disease even though symptoms such as memory loss have not yet developed. Many researchers believe that Alzheimer’s-related brain changes may begin 20 years or more before symptoms occur. In 2011, the National Institute on Aging (NIA) and the Alzheimer’s Association proposed new criteria and guidelines for diagnosing Alzheimer’s diseases.1,3-6 The new criteria propose that Alzheimer’s disease begins before the development of symptoms, and that new technologies have the potential to identify brain change before the development of symptoms. The new criteria and guidelines identify two biomarker categories: (1) biomarkers showing the level of beta-amyloid accumulation in the brain and (2) biomarkers showing that neurons in the brain are injured or actually degenerating.
Beta-amyloid imaging provides in vivo detection of fibrillar beta-amyloid (Aβ) plagues of Alzheimer’s disease.7 11C Pittsburgh Compound B (PiB) is the most well studied amyloid imaging agent.7-10 It is a radiotracer for imaging amyloid plaques using Position Emission Tomography (PET). [C-11]PiB binds with high affinity to aggregated synthetic beta-amyloid fibrils. The in-vivo retention of [C-11]PiB in brains of people with Alzheimer’s disease shows a regional distribution that is very similar to the distribution of beta-amyloid deposits in post-mortem studies.7 Compared to age-matched normal controls, Alzheimer’s disease patients have 2-3 folds greater [C-11]PiB retention on PET scans in brain areas.8,11 Many studies have shown that [C-11]PiB has a very high sensitivity of 90% for detecting beta-amyloid in patients with Alzheimer’s disease.12-15
“Pittsburgh Compound B” or [C-11]PiB or PiB for short is also known as [11C]6-OH-BTA-1 or (N-Methyl-[11C])2-(4’-methylamino-phenyl)-6-hydroxy-benzo-thiazole. It was developed by a research team from the University of Pittsburgh led by the geriatric psychiatrist William E. Klunk and the radiochemist Chester A. Mathis.16,17 [C-11]PiB is a radioactive analog of thioflavin T which was modified to include C-11 isotope. Thioflavin T is a fluorescent dye used by pathologists to identify plaques in brain tissue specimens. [C-11]PiB has high affinity to amyloid and can penetrate across the blood brain barrier with rapid clearance from background areas for maximum visualization of amyloid pathology. Through a collaboration with a researcher team in Uppsala University in Uppsala, Sweden, the radiopharmaceutical labeled with C-11 was first used in human studies in 2002.8 The name “Pittsburgh Compound B (PiB)” was given its name by the Swedish researcher team from Uppsala University.10
Challenges for production of [C-11]PiB
I.The first challenge is the high energy gamma ray and short half-life of C-11. C-11 decays by positron emission. It results in 511 keV gamma rays. The radio- chemists work with very high levels of penetrating radiation. The half-life of C-11 is 20 minutes which is very short. Therefore, a large amount of starting radio- activity is needed. To synthesise [C-11]PiB, the starting activity of C-11 at Wattanosoth Hospital is more than 2 Ci. This results in a potentially high radiation dose to radiochemists. Thus to minimize radiation exposure, everything has to take place in a hot cell. The synthesis of [C-11]PiB has been done by using automation/remote manipulation. Since, the radioactivity is reduced by 50% every 20 minutes; every step must be fast, simple and reproducible. Production must be coordinated with the PET scan. The quality control of the product has to be fast as well. With a 20 minute half-life for C-11, the C-11 radiopharmaceuticals cannot be transported long distances. Normally, the PET exam with C-11 radiopharmaceutical needs an in-house cyclotron.
II.The starting material (C-11) is a gas. It needs careful handling to avoid contamination and unintentional release into the facility. A good gas handling system for waste gas is needed to avoid any radioactive gas leak or contamination of the facility.
III.Specific activity (SA) is a major concern. Specific activity of a radiopharmaceutical is the amount of ra- dioactivity per unit mass of a compound. In the case of the specific activity of [C-11]PiB, the specific activity is the activity of [C-11]PiB divided by the mass (or molar amount) of the sum of all radioactive and stable nuclides present in the same chemical and physical form. In short, it is the ratio of radioactivity to cold mass. The contamination of atmospheric CO2 will decrease the specific activity of C-11 radiopharmaceutical significantly. The maximum theoretical specific activity of C-11 radiopharmaceutical is about 9,257 Ci/μmol. The practical specific activity of C-11 labeled radiotracers is considerably lower. Practical specific activity rarely exceeds 100 Ci/μmol and in typical practice it is about 1-10 Ci/μmol. With a C-11 labeled radiotracer having a specific activity of 1 Ci/μmol, roughly only 1 in 10,000 tracer molecules contain C-11, with the remaining containing mostly C-12.
Generally, the radiopharmaceuticals that bind to the molecular target e.g. neuroreceptors, transporters protein, and enzymes, high specific activity is required. For the radiopharmaceuticals that trace in vivo processes such as metabolism or blood flow, high specific activity is not required. Most receptor based studies require a minimum of 500 mCi/μmol at the end of synthesis in order to maintain the tracer principle. The recommendation for specific activity of [C-11]PiB is more than 300 mCi/μmol at the time of administration. The specific activity of C-11 radiopharmaceutical varies greatly among various production facilities. There are numerous factors affecting the specific activity of C-11 radiopharmaceuticals. Those factors can be grouped into 3 groups, namely the starting amount of radioactivity, the amount of C-12 contami- nation, and the amount of time in which it takes to do the chemistry.
The starting amount of radioactivity depends on the choice of cyclotron and targets, and the yield of the radio- chemistry. For example, for 10 MeV protons and 17 MeV protons, the yield of C-11 production is almost triple at higher energy.18 Therefore, with the same bombardment time; one can make more activity of C-11 with higher energy than the lower energy. The chemistry method with faster and higher radiochemical yields obviously produces higher radioactivity.

Figure 1: C-11 PRO2 autosynthesis module in a hot cell for [C-11]PiB synthesis at Wattanosoth Hospital.11
The amount of C-12 contamination is another important issue. It depends on many factors. Cyclotron targets, target body material, foils, physical target sizes and volumes also affect the specific activity. The purity of the target gas is another issue. Even with the highest purity nitrogen (UHP, 99.9999%) and the use of a hydrocarbon trap, there is still a trace of C-12. The presence of carbon dioxide (CO2) from the air containing 98.88% of C-12 and 1.12% of C-13 compete with C-11]CO2 produced from the cyclotron. Besides carbon carrier contamination in the target gas, the source of carbon contamination can come from carbon absorbed in the target chamber, CO2 penetrating in the production line and CO2 absorbed in the lithium aluminum hydride (LAH) solution. LAH solution could become a major source of CO2. LAH will absorb CO2 and will affect specific activity. High quality LAH is essential for a high specific activity product.
Production of [C-11]PiB at Wattanosoth Hospital
The production of [C-11]PiB at Wattanosoth Hospital is performed by using iPHASE C-11 PRO-2 automated C-11 radiolabelling module. C-11 PRO-2 is a dual reactor C-11 radiolabelling lab which can produce a vast variety of C-11 radiotracers. Figure 1 shows the C-11 PRO-2 module in the hot cell at Wattanosoth Hospital.
[C-11]PiB at Wattanosoth Hospital is produced by a Grignard reaction using 6-OH-BTA-0 precursor (2-(4’- aminophenyl)-6-hydroxybenzothiazole; GMP grade).
C-11 Production and labeling of [C-11[PiB
C-11[CO2] is produced by TR-19 PET cyclotron using nuclear reaction 14N(p,α)11C. TR-19 PET cyclotron is a particle accelerator producing up to 19 MeV proton beam manufactured by Advanced Cyclotron System INC (ACSI). To produce C-11, 0.1% oxygen in nitrogen with 99.9999% purity is used as a target gas. The target gas is irradiated with 19 MeV protons. The C-11 species that is formed reacts readily with the small amount of oxygen in the target gas. The primary precursors C-11 carbon dioxide ([C-11]CO2) are formed.
After the irradiation is finished, [C-11]CO2 is transferred from the cyclotron to the C-11 PRO-2 automate synthesis module in the hot cell. The [C-11]CO2 is trapped on a molecular sieve and then it is delivered to a solution of lithium aluminum hydride (LAH) in tetrahydrofuran (THF) in a reaction vial. C-11 methyl iodide ([C-11]CH3I) is produced using the “wet method” of lithium aluminum hydride (LAH) followed by hydriodic acid (HI). Then [C-11]CH3I is converted to C-11 methyl triflate ([C-11]CH3OTf) by passing through the silver triflate furnace and subsequently trapped in the high performance liquid chromatography (HPLC) loop. The [C-11]PiB is produced by labeling reaction of [C-11]CH3OTf and the precursor (6-OH-BTA-0) in the HPLC loop. The crude reaction mixture is then purified using HPLC purification for good separation. The radiochemical yield is about 18%-20% (decay corrected) with a total synthesis time of about 30 minutes after receiving [C-11]CO2 from the cyclotron. In routine synthesis at Wattanosoth Hospital, the starting activity of [C-11]CO2, measuring at the molecular sieve, is about 1.8-2.3 Ci. [C-11]PiB produced at the end of synthesis is about 200-250 mCi. The specific activity of [C-11]PiB at the end of synthesis is about 2-5 Ci/μmol. The brief equation for radiosynthesis of [C-11]PiB is shown in Figure 2.
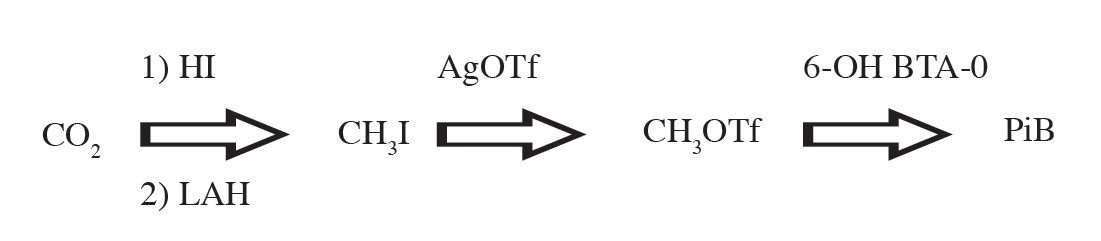
Figure 2: Main chemical reaction for [C-11]PiB synthesis
In order to minimize the presence of C-12 along the process of irradiation, transferring and synthesis of radio- pharmaceuticals labeled with C-11, the following method is applied at Wattanosoth Hospital.
For [C-11]CO2 Production
For [C-11]PiB Production
When starting the production here, the initial specific activity of [C-11]PiB was less than 300 mCi/μmol. Searching for an improvement of specific activity from extensive literature and expert’s opinions along with experimenting with a lot of factors, finally the specific activity of [C-11]PiB was improved greatly. After applying the above protocol, the specific activity of [C-11]PiB becomes more than 2.5 Ci/μmol. Since the technical details are beyond the scope of this article, these details can be found in upcoming articles from our group. [C-11]PiB PET imaging in Wattanosoth hospital has been servicing patients with problems with dementia since November 2012. The PET images for [C-11]PiB for normal control and Alzheimer’s patients are shown in Figures 3 and 4.
Early detection of Alzheimer’s disease is a key goal of current research to slow the progression of the disease. At present, the onset of Alzheimer’s disease cannot yet be stopped or reversed. However, an early diagnosis allows patients with dementia and their families a better chance of benefiting from treatment, chances to participate in clinical drug trials and more time to plan for the future. With the effort to improve the quality of radiopharma- ceuticals, the current [C-11]PiB production at Wattanosoth Hospital can service 2-3 patients a day requiring a PET examination.
The author is very grateful for the support from the executive of the Bangkok Hospital Medical Center and Wattanosoth Cancer Center. Without the support from the executive, the development of new PET radiopharma- ceuticals will not be possible.
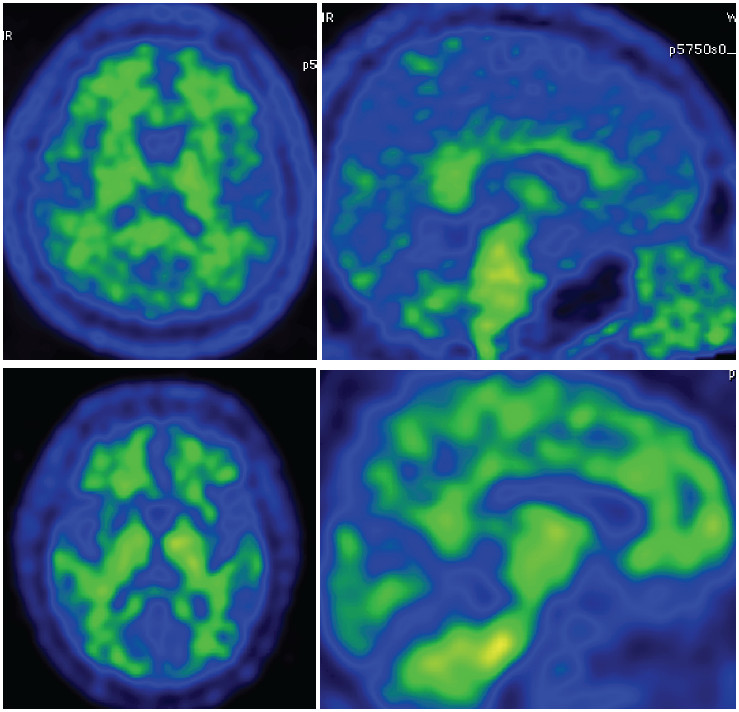
Figure 3: PiB-PET image in normal subject shows no accumulate of beta-amyloid over the cortex area.
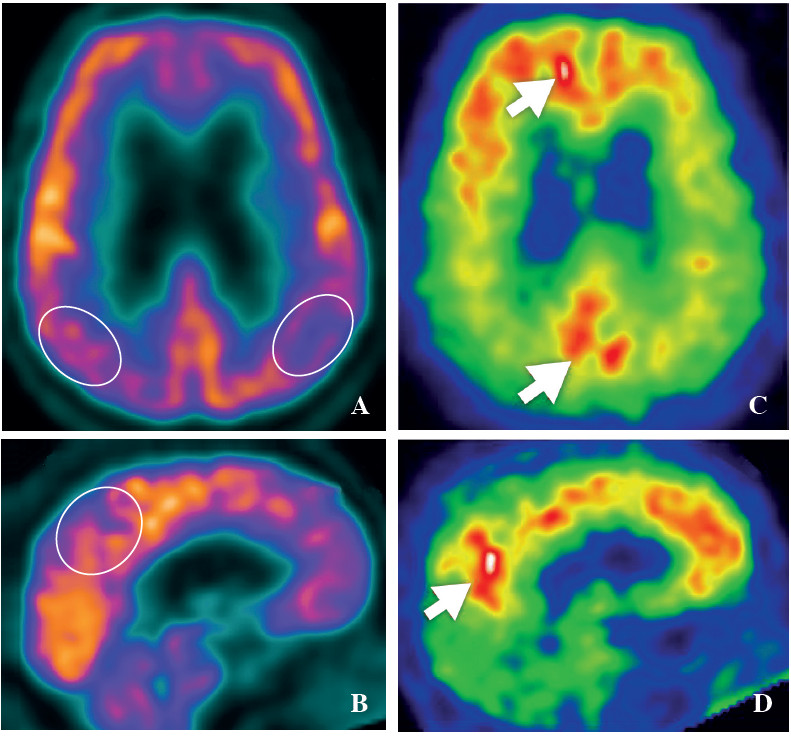
Figure 4: FDG-PET and PiB-PET image in Alzheimer’s dementia patient. The FDG-PET reveals hypometabolic activity over bilateral parietal lobe, posterior cingulate and precuneus (A, B), whereas PiB-PET shows accumulation of beta-amyloid over the cortex areas (C, D).