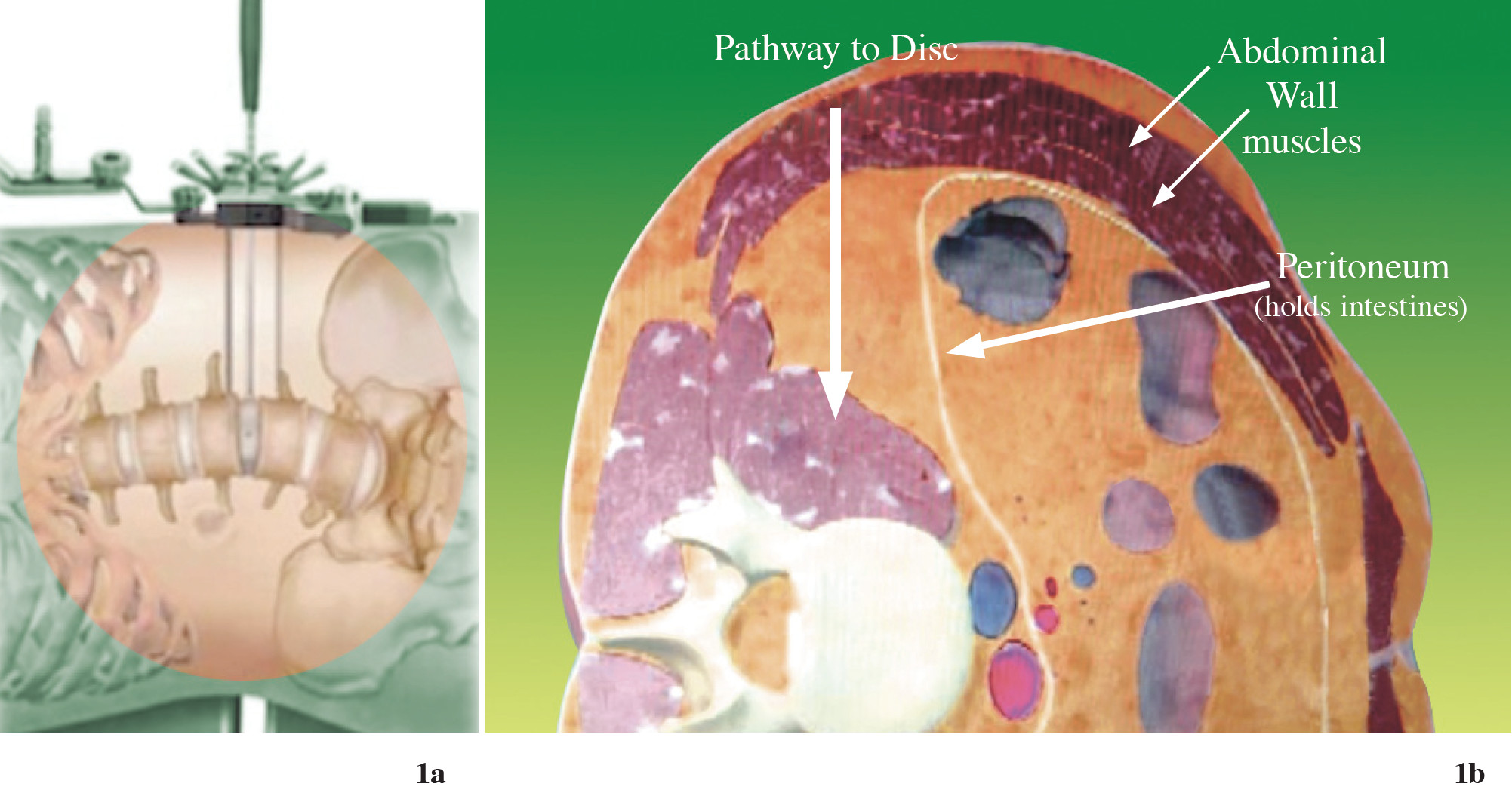
Figure 1a-b: Shows the new lateral trans-psoas approach may be achieving the advantage of lumbar spinal fusion. The retractor passes through loose retroperitoneal space directly to that which needs fusion.5
The true lateral trans-psoas lumbar interbody fusion procedure is a modification of the retroperitoneal approach to the lumbar spine, which uses a tubular dilator/retractor system and was first reported in the literature by Ozgur, et al. in 2006.1-4 Other terms commonly used to refer to this technique include lateral lumbar interbody fusion, lateral transpsoas interbody fusion (Figure 1a-b), direct lateral interbody fusion (DLIF®, Medtronic Sofamor Danek, Inc., Memphis, TN, USA) and extreme lateral interbody fusion (XLIF®; Nuvasive, San Diego, CA, USA). DLIF and XLIF are different in their instrumentation/ retractor systems, but not in their approach. Even though these minimally invasive lumbar fusion technologies are new and have limited literature reporting on outcomes, this procedure continues to gain popularity among spine surgeons in several countries. Most of the reports show impressive results in clinical and radiographic terms, with few complications. Especially in patients with multilevel degenerative discs which have to be corrected, as in degenerative scoliosis, the advantages of this procedure seem to be clear.
Compared with anterior lumbar procedures (ALIF), DLIF shows superiority in terms of not requiring an access surgeon, eliminating the need to violate or retract the peritoneum and obviating the need for great vessel mobilization.6 It also eliminates the risk of retrograde ejaculation7 in males due to inadvertent superior hypogastric nerve plexus disruption during anterior approach procedure and there is reduced incidence of ileus post operation.
One of the major advantages of DLIF is the fact that the supporting back muscle is still intact. The lack of need for bony resection on the posterior column when compared with posterior fusion means that the posterior ligamentous complex (“the tension band of spinal column”) is still functioning during body flexion and extension (which may reduce the chance of adjacent problem). The intact anterior and posterior longitudinal ligament on vertebral column allows the cage to be fixed in the center of body and for better correction of spine in sagittal and coronal alignment, preventing dislocation of the graft and cage, reducing blood loss and operative time (as compared to other approaches in many reports), and reduced postoperative hospital stay and analgesic requirements.
In our center (Bangkok Spine Academy), the DLIF operation has been commenced since February, 2012 (Figure 2). For the purpose of this article, we would discuss the indications and candidates for this operation, the surgical steps, and include preliminary results of the first 12 cases with details of blood loss, complications, radiographic evaluation and early clinical outcome.
Contraindications
During preoperative consultation, each patient was informed of all the surgical options. A complete discussion and description of the approach technique was described to all patients interested in this procedure.
Indications and surgical candidates
Trans-psoas lateral interbody fusion has the same surgical indication as posterior lumbar decompression or spinal fusion in patients who have axial back pain and/ or radicular leg pain. For example, patients who have axial back pain from degenerative disc disease, which requires total removal of the disc and segmental spinal fusion or the patient who has leg pain from spinal stenosis due to segmental disc degeneration and ligamentum flavum thickening. Especially those who have disc bulging or collapse that are responsible for corresponding foraminal and lateral recess stenosis (but excluding central canal stenosis) are good candidates for this procedure. The total disc removal and space distraction by the cage will open the foramen and both lateral recess directly. Lumbar spondylolisthesis “slipped vertebra” grade 1 and 2 with or without nerve root compression are also the candidate for this procedure. The large intervertebral DLIF cage that was inserted into the disc space will distract the disc height and also the automatic reduction of the slipped vertebra in to normal sagittal alignment and also indirect decompression of the nerve root within spinal canal and its foramen. Lumbar segmental instability such as degenerative scoliosis is also the candidate for direct lateral interbody fusion. By this surgery, the segmental coronal tilting automatically reduces due to the insertion of the large intervertebral DLIF cage and when done multilevel, the abnormal global coronal alignment will correct into acceptable balance. Furthermore, it can decompress the neural canal and foramen indirectly in each level to eliminate patient leg pain.

Figure 2: The occasion of the first operative demonstration of DLIF technique in Thailand on February 17, 2012 at Bangkok Spine Academy, Bangkok Hospital. The surgeon who was invited to perform this operation is on the middle, Dr. Robert Watkins, Jr. from Marina Spine Center, Los Angeles, USA.
The contraindications of direct lateral interbody fusion technique are congenital spinal canal stenosis, patients who have severe osteoporosis and auto-fusion in between each level that cannot be separated. All L1-2, L2-3, L3-4 disc spaces and the majority of L4-5 discs (lying higher than the bony iliac crest level) are candidates for the procedure. For L4-5 and L5-S1 (lying below the iliac crest level) the procedure is contraindicated.
Patient selection
The enrolled patients in this study had the indication for spinal fusion and/or spinal decompression. All had lesions from L1 to L5 without symptomatic lesion at L5/S1.
Indications
Contraindications
During preoperative consultation, each patient was informed of all the surgical options. A complete discussion and description of the approach technique was described to all patients interested in this procedure.
Data for this study was obtained through retrospective chart reviews and concurrent follow-up of patients who underwent DLIF surgery by a single spine surgeon (T.B.) at Bangkok Spine Academy. Outcome data were obtained prospectively preoperatively and at each visit postoperative through self-administered questionnaires. The roentgeno graphic data was obtained and calculated by another orthopedic doctor (K.J.).
The clinical charts were reviewed to identify the complications and outcomes. Chart review included compilation of demographics (age, gender), symptoms and diagnosis, surgical details (levels treated, instrumentation used, blood loss, operative time, complications), hospital stay, additional procedures, results of physical exams, lateoccurring complications and patient complaints, prospectively collected back and leg pain scores (Visual Analog Scale, VAS). The radiographic measurements were taken before and after the operation to assess change in the sagittal and coronal plane alignment of the individual operated disc level, overall lumbar spine, and lumbar scoliotic curves. The radiographs were also analyzed for vertebral fracture, end plate indentation, correction of sagittal and coronal plane, vertebral slip correction in sagittal and coronal plane in each level, nerve root injury, implant malposition, wounds and other possible complications.
Surgical technique
Under general anesthesia, the patient is prepared in the same manner as normal spine surgery. The IV line and urinary catheter are placed. The needle recording electrodes are placed in the innervated muscles in the legs to monitor the affected nerve roots during the procedure, then patient is transferred to operating table and placed in a true 90 degree right lateral decubitus position with the left side elevated and taped in this secured position, bending laterally in such a way as to increase the distance between the iliac crest and the rib cage, making sure the patient’s hip is in flexion position for relaxing the psoas muscle and nerve. A cross-table anterior-posterior (AP) image helps to confirm the true 90 degree position (Figure 3).
After aseptic treatment of the skin, AP and lateral fluoroscopic images are used to identify the lumbar disc’s mid-position. A marking point is made on the patient’s lateral side, overlying the center of the affected disc space. Through this mark, a small incision will be created transversely. The surgeon uses his finger to perform blunt dissection, passing through a lateral abdominal muscle. The layer of muscles are spitted along their fibers until the surgeon’s finger can pass the transversus abdominis fascia, then the fat tissue in retroperitoneal space is exposed. The peritoneum and its visceral content are protected and retracted anteriorly with the angle retractor. The retroperitoneal space is bluntly dissected in straight direction until the anterior surface of psoas muscle is exposed (Figure 4).
After the fluoroscope is checked for the correct level, the PAK probe (NIM® X-PAK Probe: connected to the electrophysiological monitoring and using triggered and free running electromyography) is introduced directly into the target disc, making sure the abdominal contents are not in the way. This monitoring method is used to reduce the risk of injuries to the lumbosacral plexus when accessing the disc space through the psoas muscle. After the PAK probe is in the correct position, the guide wire is introduced into the target disc.
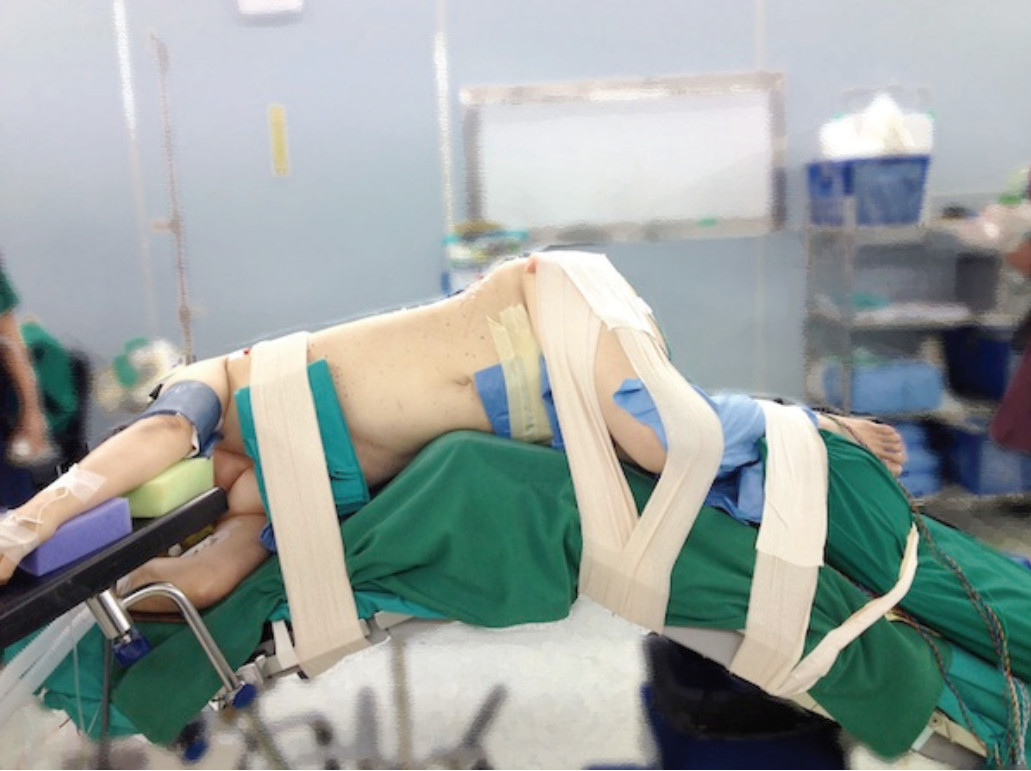
Figure 3: Shows the position of the patient for DLIF procedure; mostly on the right lateral decubitus. The table is positioned at maximum bending to keep lumbar inter vertebral disc opened.
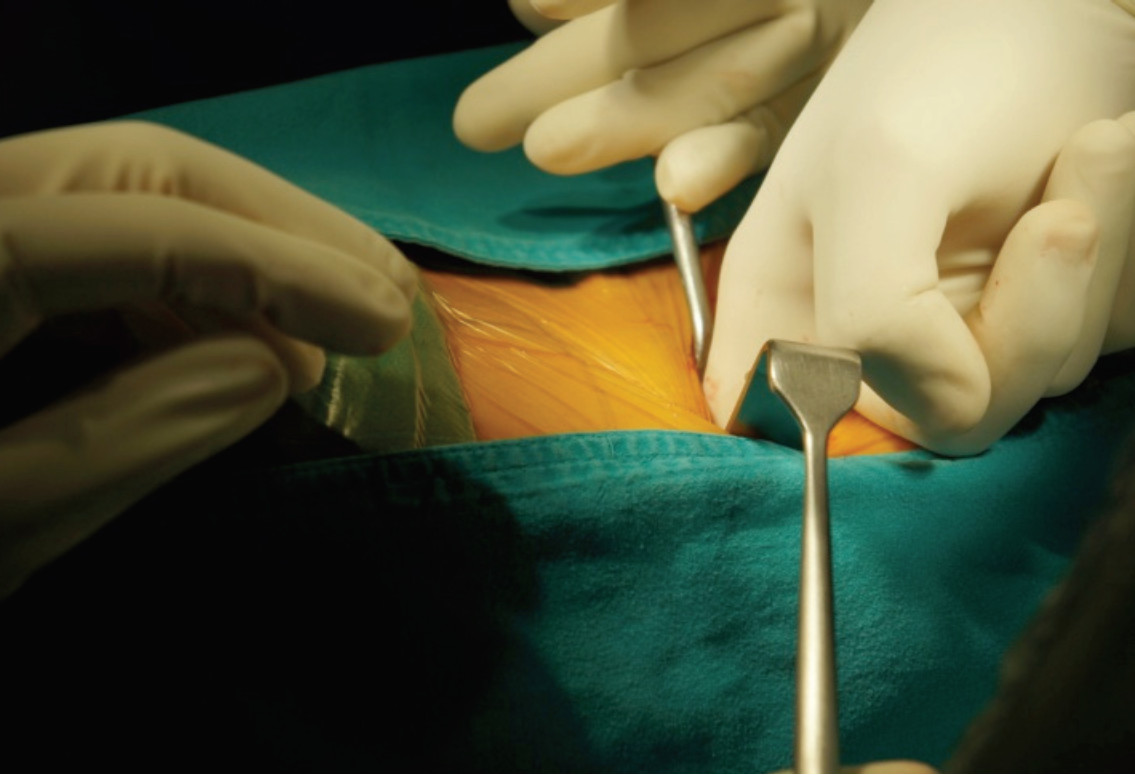
Figure 4: Shows the approach performed by bluntly dissection on the left flank. The finger is passed anterior to the psoas muscle until reaching the tip of spinous process. Make sure the abdominal content and peritoneum are retracted to the front.
The probe is removed, and then sequential dilators are introduced into the psoas muscle in the direction of guide wire, attached directly to the lateral aspect of disc. The ball tip EMG probe (NIMS-Spine ball tip probe) is used to check the final dilator. Make sure there is no lumbosacral plexus nearby this dilator (Figure 5a-b). After that, the proper DLIF retractor can be inserted through the psoas muscle and fixed.
After the dilator is removed, the position of this retractor can be checked by fluoroscope, both AP and Lateral. The ball tip EMG probe is used to check the lumbosacral plexus in all the areas of tubular retractor. After the light illumination fiber optic is attached, the surgical field inside the tube should be inspected to ensure that there are no neural structures in direction of working area. The bleeding is stopped by the bipolar coagulator.
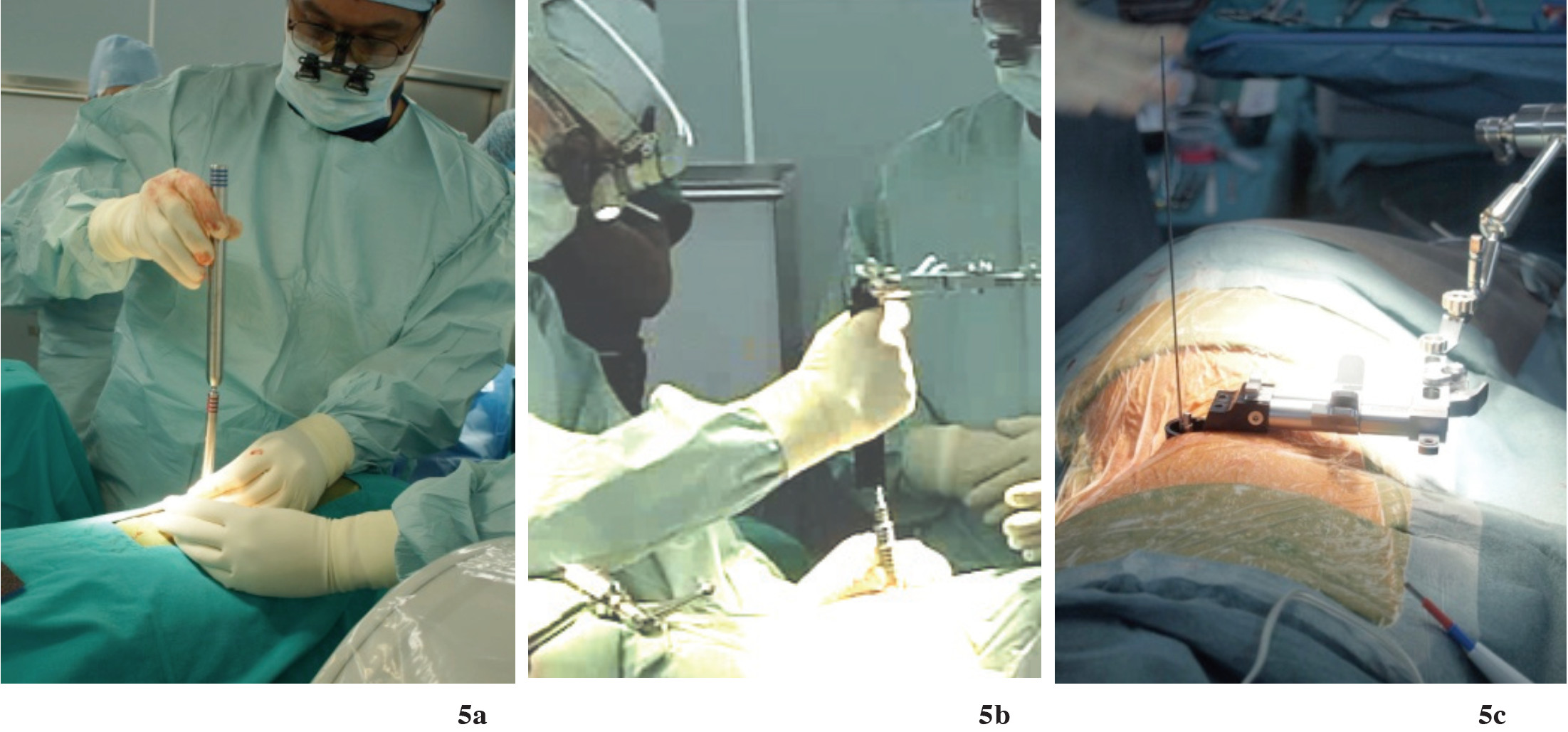
Figure 5a: Shows the sequential dilator (5a) is bluntly passed through the retroperitoneal approach directly to the inter vertebral disc until reaching maximal size.
Figure 5b-c: The tubular retractor is securely placed in the tract of dilator. Then expose the intervertebral disc. The exposure is around 1 inch diameter; use intra-operative neuromonitoring to make sure it is safe and will not cause nerve injury (Figure 6).
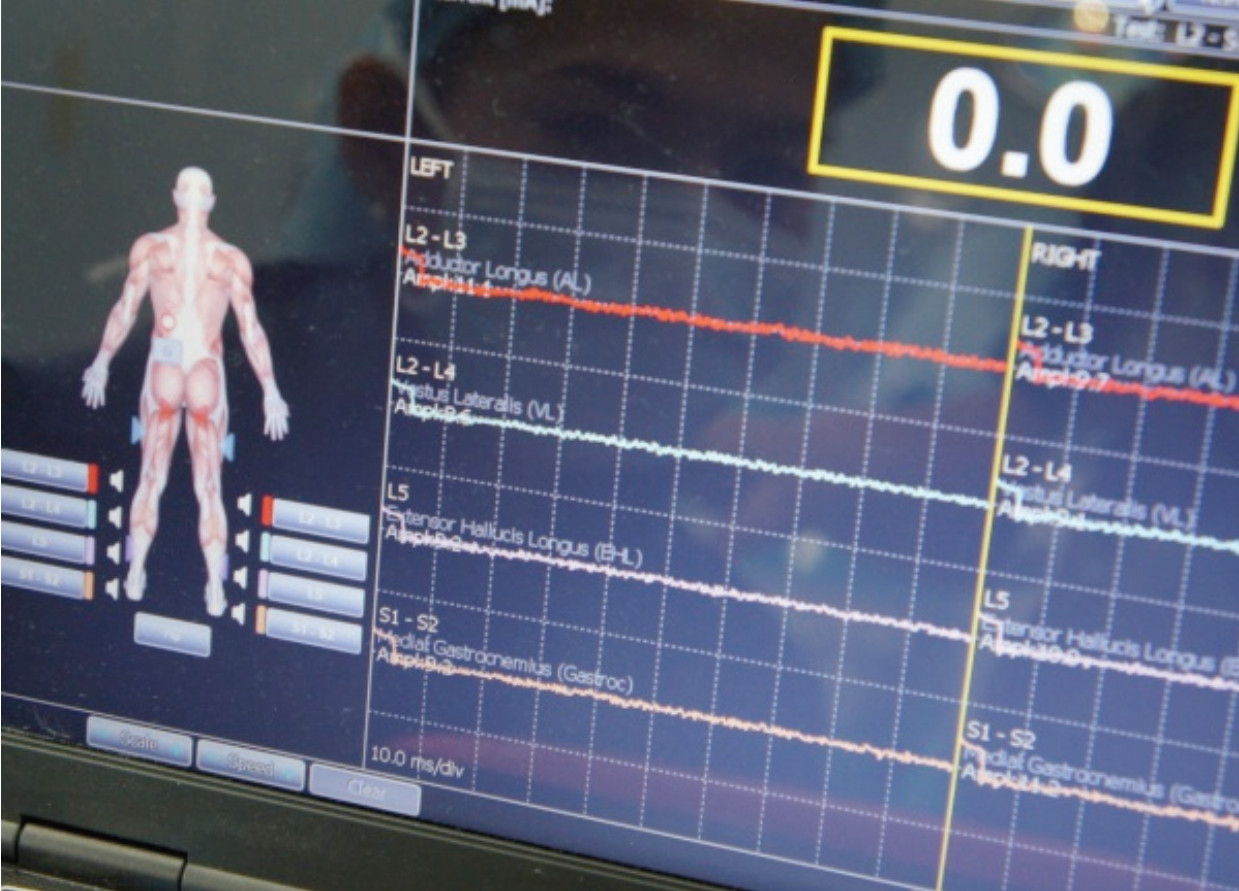
Figure 6: Shows the intra-operative neuromonitoring in use to evaluate the lumbosacral plexus, making sure the neural structure is safe from interference by instruments
The intervertebral disc is opened as a rectangular window, like the size of cage. The disc material is removed by a disc punch. A Cobb elevator is used to separate the disc material and cartilaginous end plate from bony end plate of vertebral body. Under fluoroscopic control using AP imaging (Figure 6a), the Cobb elevator is used to release the annulus on the contralateral side and cross just beyond the disc space. The end plates are then meticulously prepared with the disc shaver, rasps and curettes until the bone is bleeding in order to have a good fusion bed.
We use several serial trial spacers to elevate and trial for the correct size of the implant, as compared to the normal disc. After the final trial, the correspondingly sized DLIF cage (Clydesdale cage, Sofamor Danek, USA) was filled with bone graft/bone graft substitute extender and/or biologic material. (rhBMP-2 “infuse®” or demineralized bone graft “Grafton®”) see figure 7. We then inserted the implant and impacted it into the disc space to the correct position under fluoroscope guidance (Figure 8c-d).
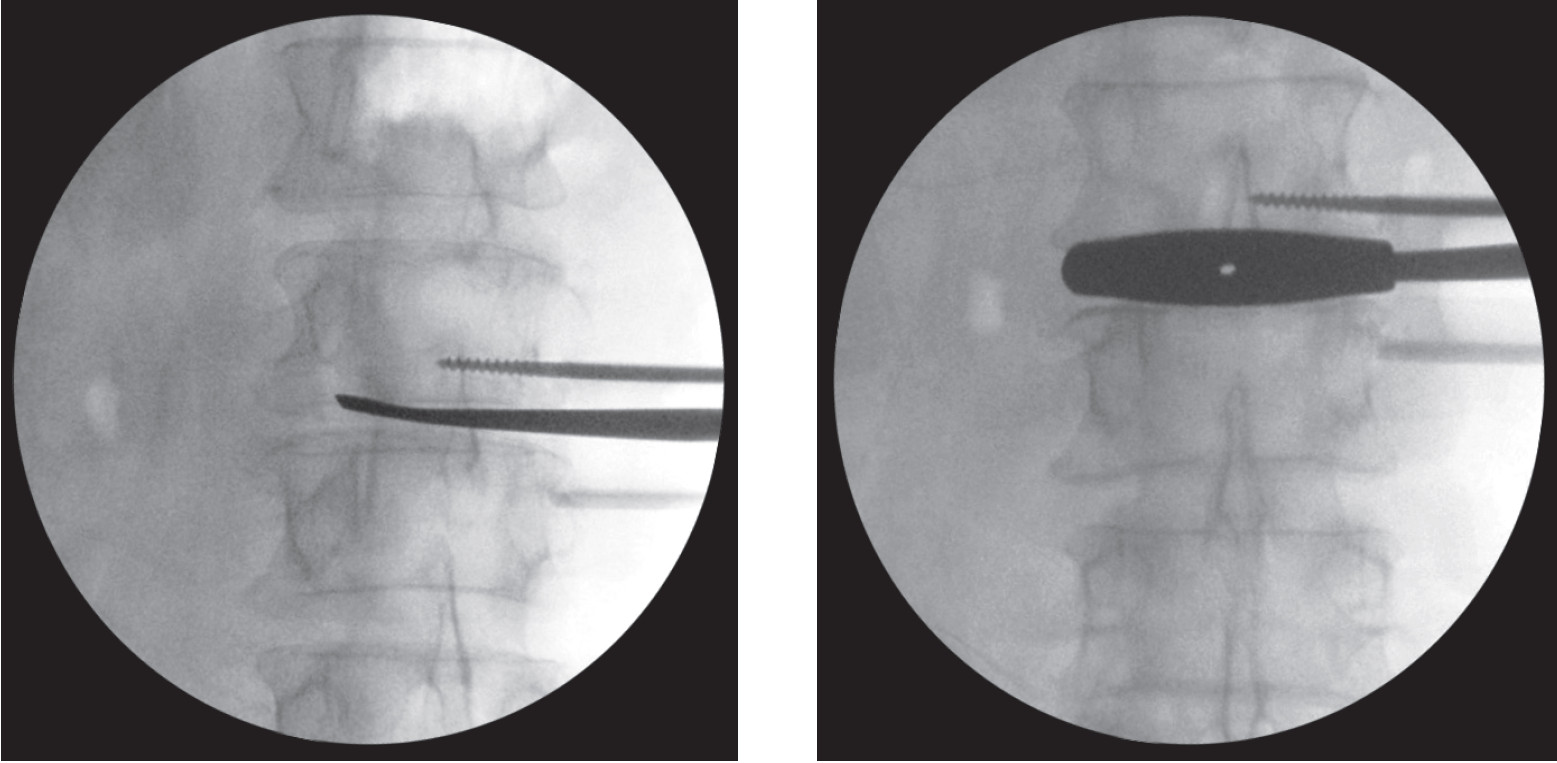
Figure 6a: Shows the exposed intervertebral disc and disc material is totally removed using the Cob elevator and Curette.
Figure 6b: Shows serial trial spacer inserted into intervertebral disc space to the correct size and position. Make sure it’s well fitted with the verte brae and distract the spinal column to the normal alignment.
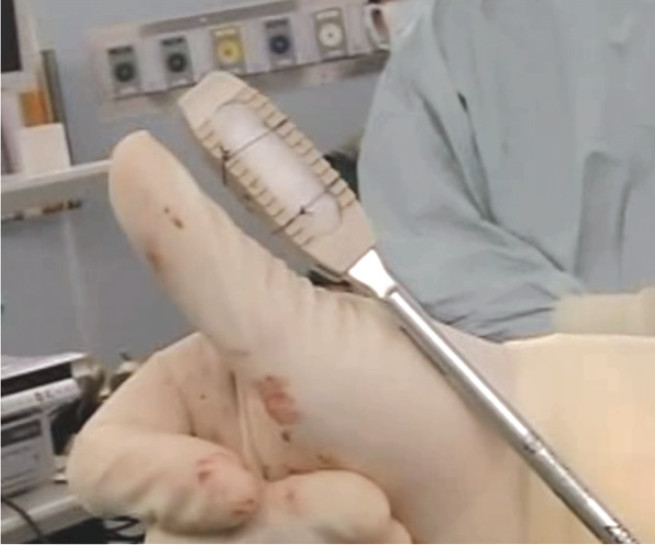
Figure 7: The DLIF cage compared to the size of Thumb. Note this cage is bigger than the cage used in posterior approach such as TLIF or PLIF, so the biomechanics support the body weight better. Note the white material inside this cage is the bone forming agent “rhBMP-2 (infuse®)”.
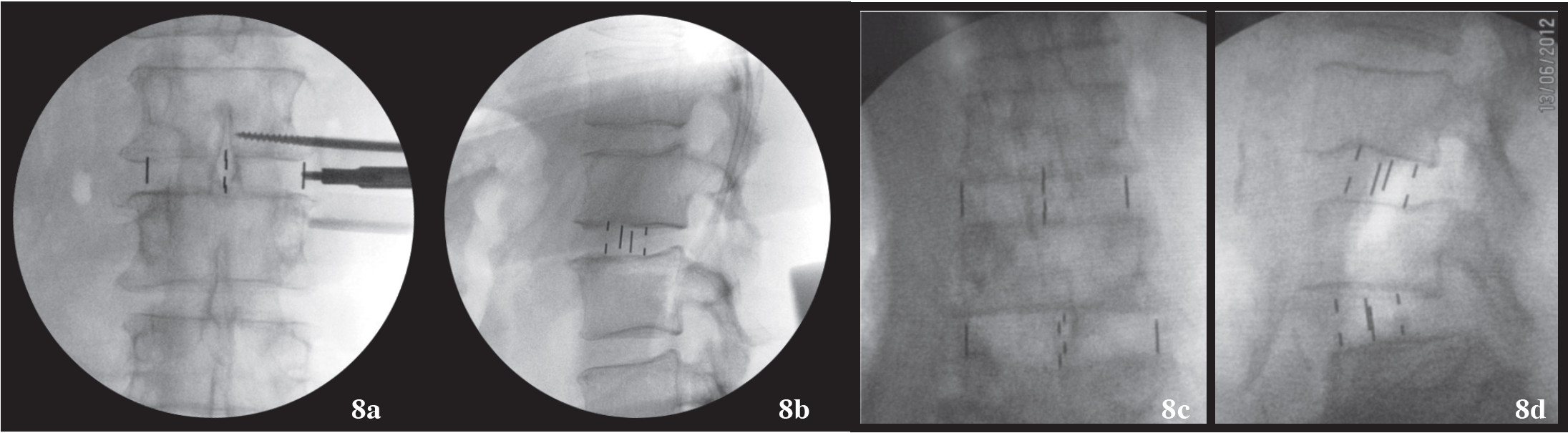
Figure 8a-b: Shows the intervertebral cage (DLIF cage) as finally inserted into the space and checked under fluoroscopy under AP and Lateral fluoroscope views. Note the minimal breaching of the superior end plate that is almost asymptomatic.
Figure 8c-d: Shows two levels of DLIF where cage is in proper position. Note the slippage of vertebra is markedly improved and the intervertebral space is nicely distracted.
Figure 9: Shows the size of flank wound after finish DLIF operation is 3-4 cm. One incision can account for 2 levels being fused, meaning this operation is indeed minimally invasive. No need for any drainage from this wound.
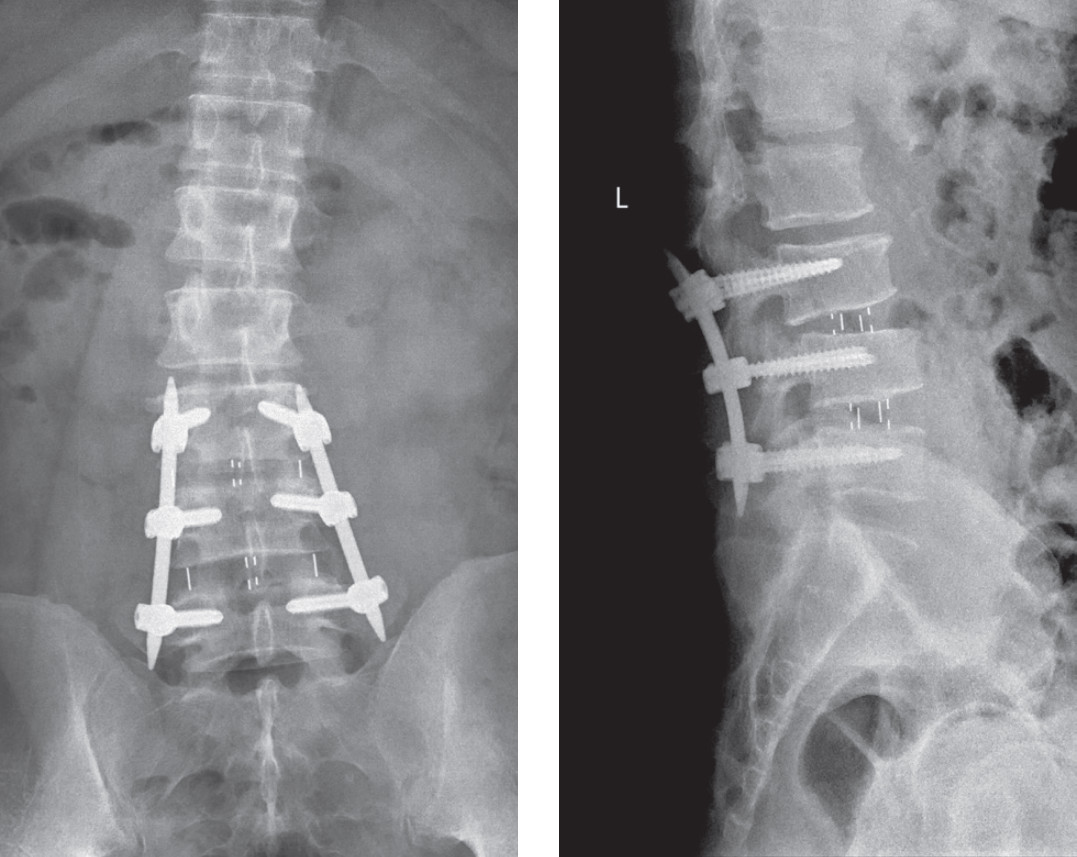
Figure 10: Shows after DLIF procedure is finished. The augmentation with posterior pedicular screws by percutaneous method is recommended for maximal stability of the vertebral column.
Some literature reported performing stand-alone DLIF procedures, but we made sure that we augmented the stability of the vertebral segment in all of our patients by percutaneous pedicle screws insertion (Sextant®: Medtronic Sofamor Danek for 1 level fusion and Apollo®: Orthopesia Co., Thailand for 2 or more levels), in order to achieve maximal stability.
Demographic and Clinical Data
All patients had DLIF procedure performed for lumbar degenerative disc disease, spondylolisthesis, or Adult scoliosis. Most cases had indications of back pain with radiation to the leg from nerve root compression. Two cases (16.6%) had back pain only from single level severe disc degeneration with sign of instability and unresolved by non-surgical treatment. Five cases (41.7%) were diagnosed of degenerative scoliosis and another five (41.7%) had degenerative spondylolisthesis that did not respond to non-surgical treatment.
As for the distribution of age and gender, male and female were equal. The average age was 60 years old, (range:37 to 82).
The level of spine that was affected and operated on included L1-2 disc space to L4-5. The spinal levels that were mostly operated on were L4-5 and L3-4 respectively.
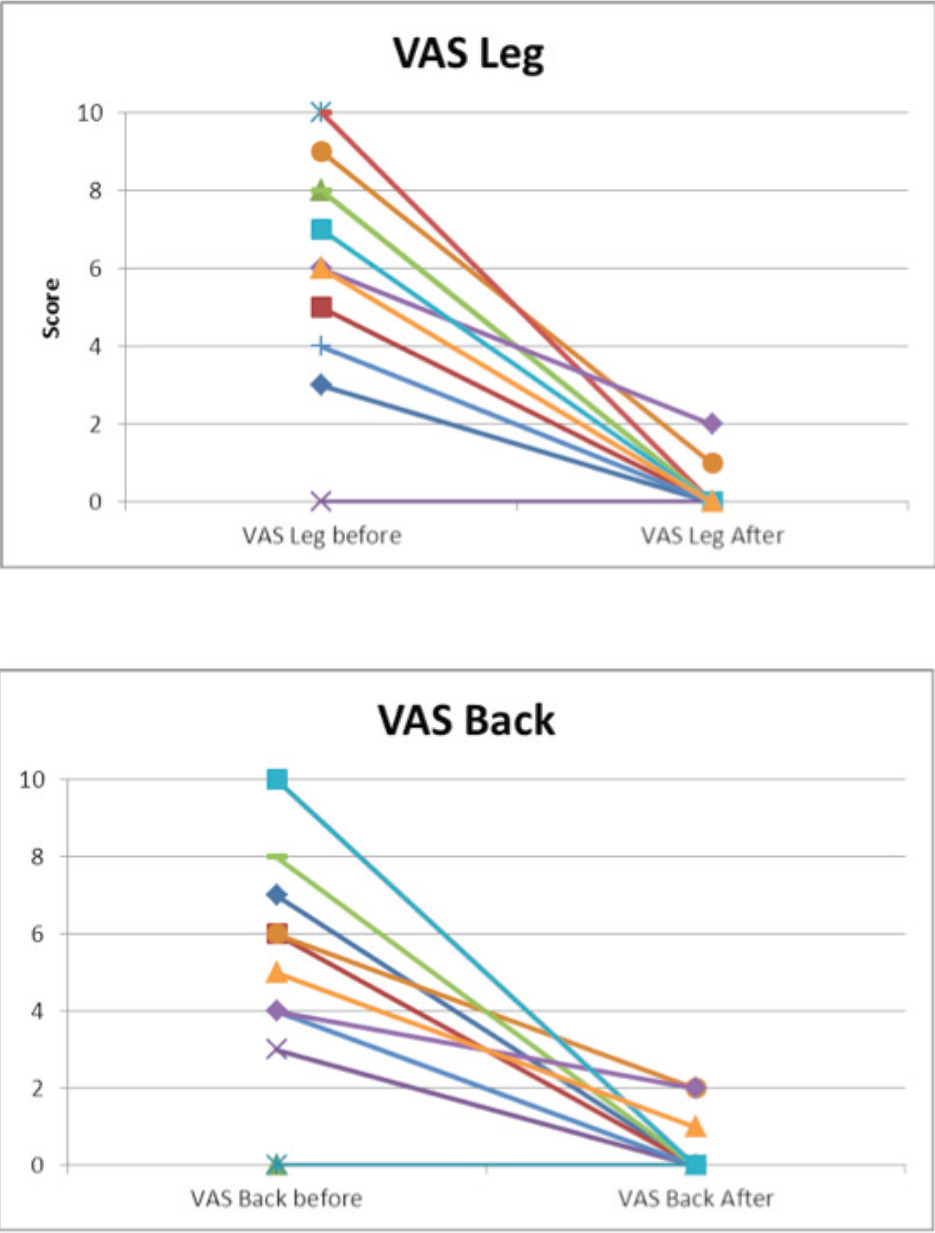
Mean VAS before surgery was 6.3 for leg pain and 5.3 for back pain. Some patients had only one symptom, either back or leg pain.
Because of the preliminary report of DLIF operations in our center, the Oswestry Disability Score that has been collected before operation and then compared with post operative status at 6 weeks, 3 months and 6 months are not yet completed.
Demographic and Clinical Data are shown in Table 1.
Table 1: Demographic and Clinical Data.
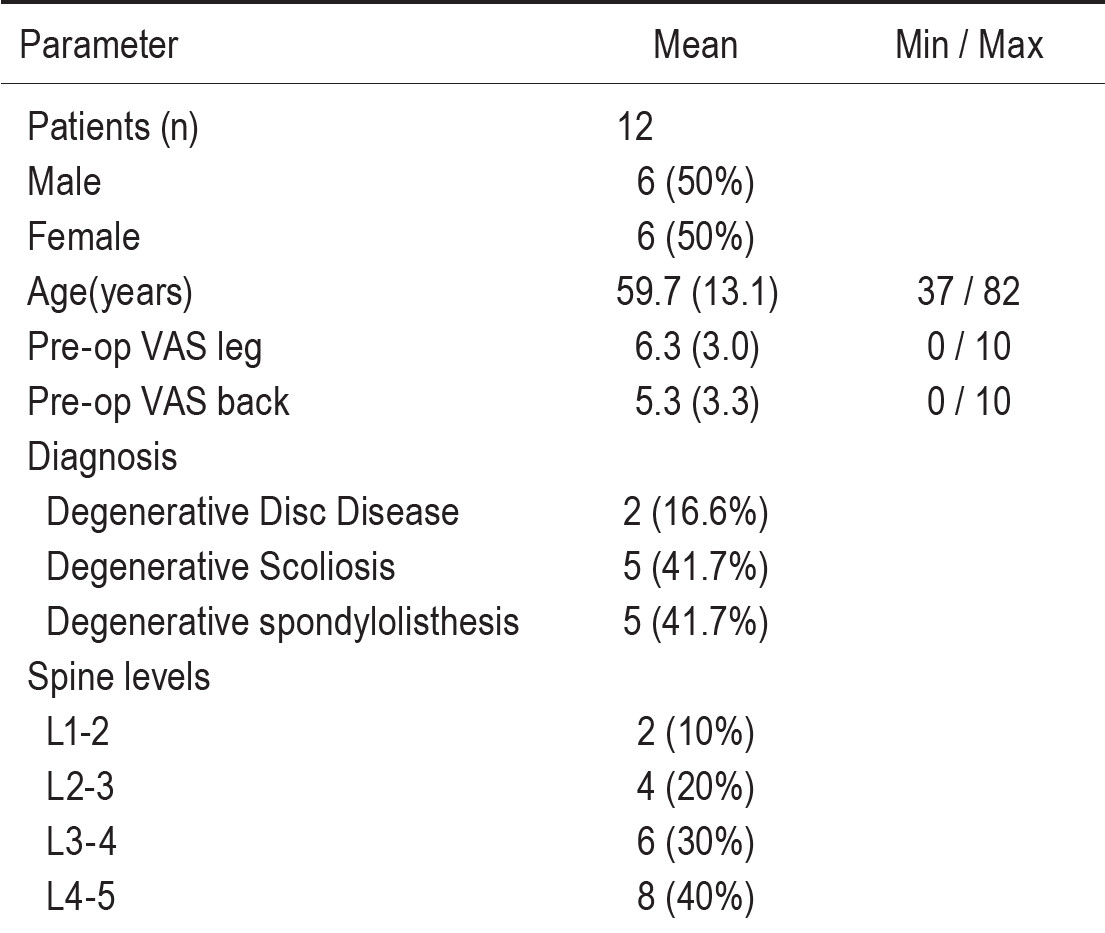
Table 2: Radiological parameters

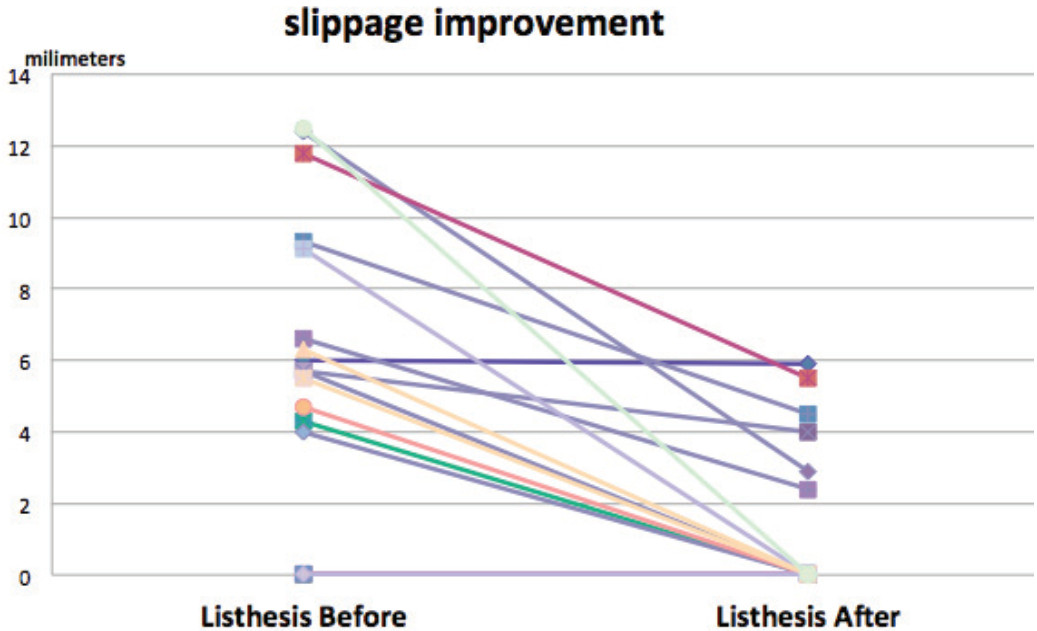
Operative and Clinical outcome
Surgical duration was measured by skin to skin time. Mean operative time was 70.6 (±17.7) minutes (mean ± standard deviation). Minimal surgical duration was 41.7 minutes for each level and maximum 110 minutes. The operative time seemed to be faster after we passed the second case. Intraoperative blood loss averaged 81.7 (±27.6) mL. Minimum blood loss for each level was 30 mL. and maximum was 130 mL. Blood loss mostly occurred during vertebral end plate preparation.
There were no intraoperative complications. The bleeding during operation was much less than conventional surgery. Because of the nature of this operation is indirect reduction, the intra-operative complication that comes from opened surgery such as dura tear does not exist. Neurological examinations showed one patient developed Psoas weakness with anterior thigh numbness, both conditions resolved within 2 weeks.
Clinical scores in patient-reported questionnaires (VAS back, VAS legs) showed fast and lasting pain relief and improvement in daily activities. Mean VAS back scores decreased from 5.3 (3.3) to 0.4 (0.8). For leg pain assessment, mean VAS scores decreased from 6.3 (3.0) to 0.3 (0.6) at the 2 weeks visit.
Radiographic outcome
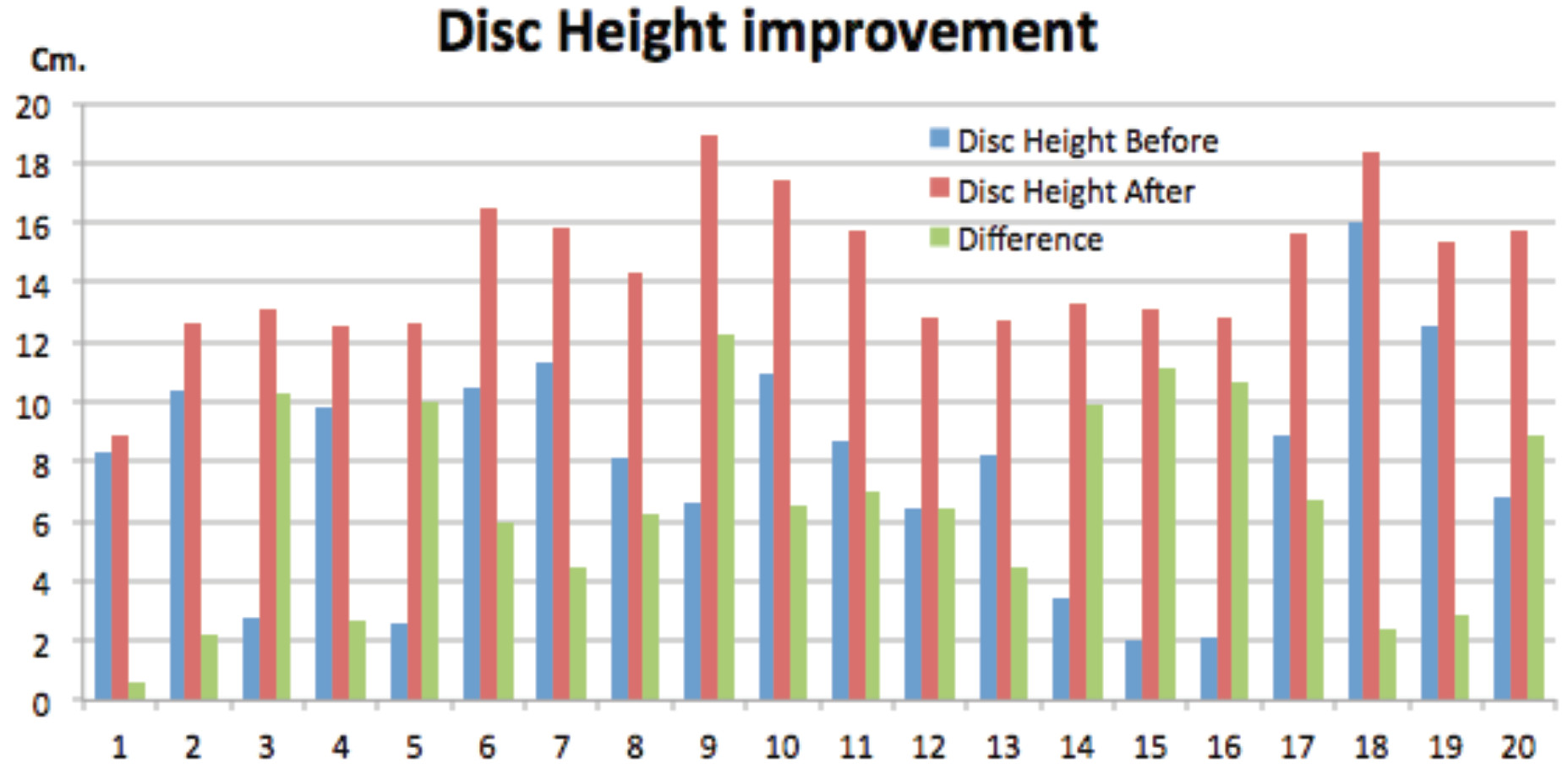
There was a mean correction of 7.9 to 3.2 degrees in segmental coronal plane in all instrumented levels. There was significant change in the overall coronal plane alignment of the lumbar spine. Increasing disc height at middle column 7.0 (0.3) mm.
Low back pain is very common. Even though much back pain comes from non specific problems, many conditions still need to be treated by surgical intervention, for example, neurological compression by pathological structures of lumbar spine such as disc herniation or back pain due to degeneration or instability of the vertebral segments. The most common surgical intervention for neurological compression is surgical decompression, or “laminectomy” procedure9-11 but half of the cases need a more complicated procedure known as “spinal fusion” to eliminate segmental instability, reduce back pain, maintain spinal canal in such a way as nerves do not get compressed despite different body positions used in daily life activities. Spinal fusion can also improve spinal alignment for normal human posture.
Lumbar spinal fusion has been recognized as a treatment option for symptomatic spinal instability, spondylolisthesis, and degenerative scoliosis. The aim of fusion is to reduce back pain by reducing the motion of segments; the higher the fusion and possibility of achieving a better fusion rate, the better the patient satisfaction. When spinal fusion technique first began to be used, it was non-instrumented and bone was grafted on the posterior surface of lumbar spine (Figure 10). However, we now know that the non-instrumented spinal fusion rate is less than that achieved from anterior interbody fusion. (Figure 11).11-14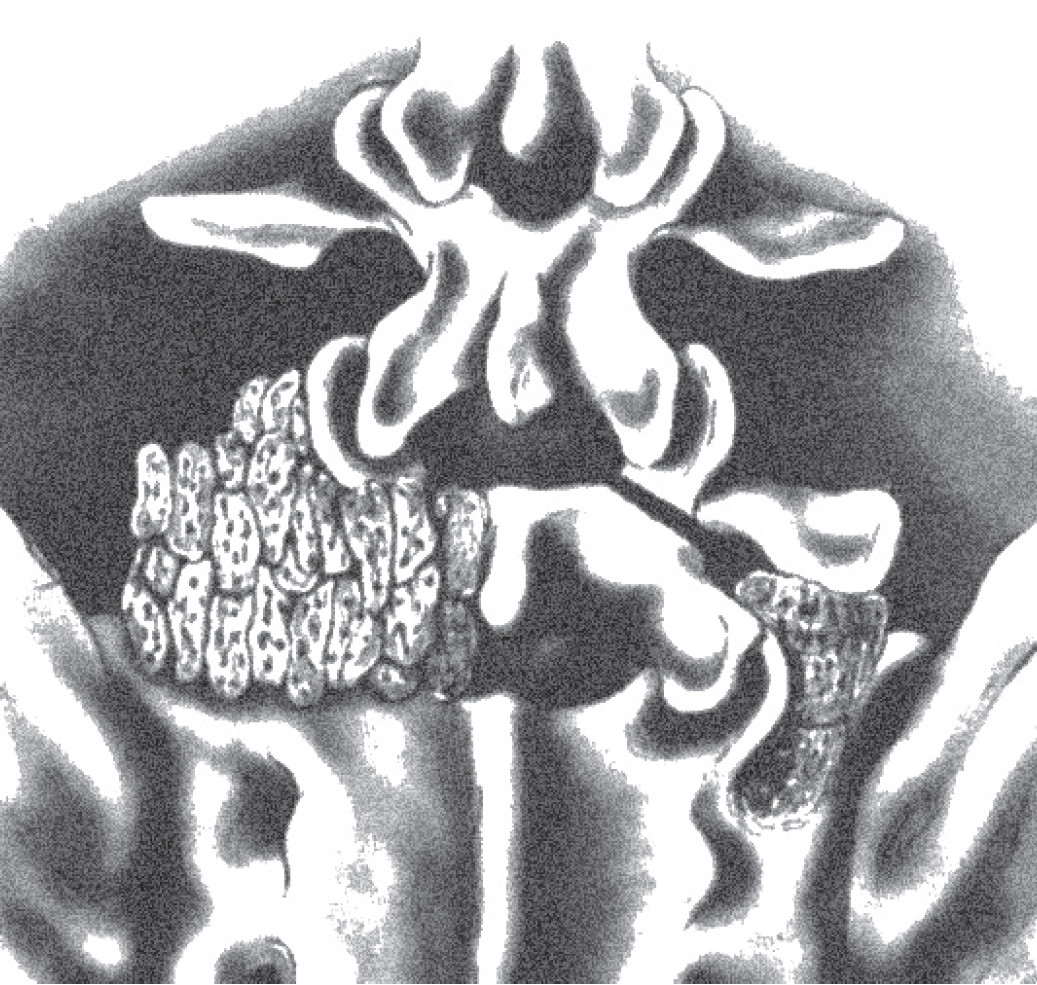
Figure 10: Shows how to do posterior fusion by placing the ship bone graft posteriorly. The biomechanics, rate of fusion, stability was acceptable but inferior to fusion from anterior.
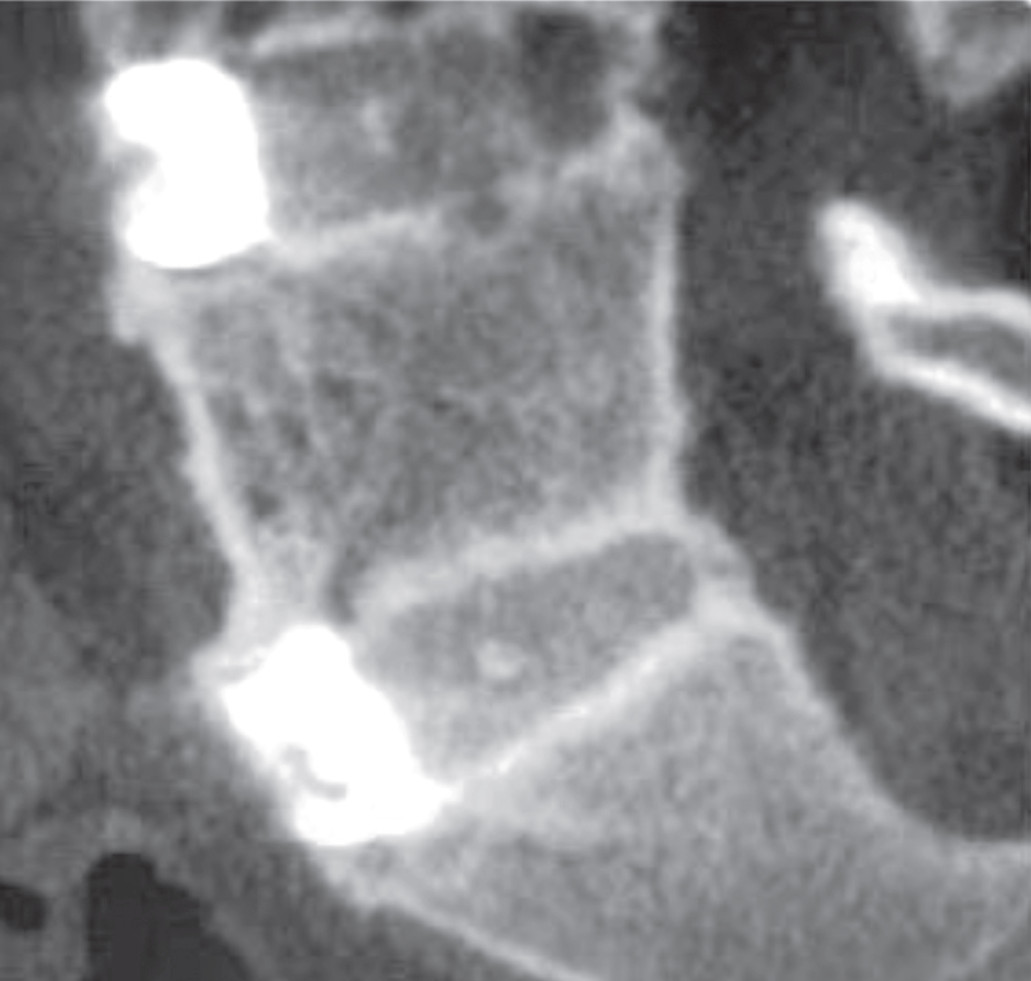
Figure 11: Anterior lumbar interbody fusion (ALIF) shows the complete fusion at L45 AND l5-S1. Anteriorly fusion shows superiority in terms of biomechanics, rate of fusion, stability and alignment when compared posterior fusion.
Spinal fusion technologies have continued to develop and improve, from non instrumented fusion that has a limited improvement of spinal alignment to instrumented spinal fusion that can improve both fusion rate and spinal segmental alignment.11-19 Most surgeons agree that the spinal fusion in the anterior column of spine is better than posterior fusion, as regards fusion rate, ability to keep the segment in normal spinal alignment with both coronal and sagittal balance. Including the overall clinical outcome, anterior column fusion shows a superior result. The only problem of anterior fusion is the difficulty in approach from the anterior abdominal wall and the accessibility through the great vascular bundle in front of the vertebral column (Figure 12).19-22, 25-26
Thus the development of “anterior fusion from the posterior”, PLIF (Posterior Lumbar Interbody Fusion) or TLIF (Transforaminal Lumbar Interbody Fusion), which achieve the benefits of anterior column fusion but eliminate the difficulties of anterior approach (Figure 13). The surgeon can do anterior fusion from the small opening site at posterior wall of intervertebral disc during posterior decompression and discectomy. The ship bone graft is impacted in the space and the disc height can be restored by small “cage” instruments before the spinal segment is held by inter-pedicular screw constructs.
Thus the development of “anterior fusion from the posterior”, PLIF (Posterior Lumbar Interbody Fusion) or TLIF (Transforaminal Lumbar Interbody Fusion), which achieve the benefits of anterior column fusion but eliminate the difficulties of anterior approach (Figure 13). The surgeon can do anterior fusion from the small opening site at posterior wall of intervertebral disc during posterior decompression and discectomy. The ship bone graft is impacted in the space and the disc height can be restored by small “cage” instruments before the spinal segment is held by inter-pedicular screw constructs. back muscle denervation, excessive blood loss, iatrogenic excessive removal of stabilizing bone and ligament structures and also increased post operative pain have all been reported. The overall spinal correction results by PLIF and TLIF are still limited in their ability to restore disc height, or improve the sagittal and coronal alignment. Furthermore the success rate of fusion is also affected by the size limits of bone graft and cage that can be inserted in the small annular hole.23-24
As spinal surgery evolves to “minimally invasive technologies”2-5 (Figure 14), the primary goals are to minimize paraspinal muscle retraction and dissection in order to reduce blood loss and post operative pain, accelerate recovery period and improve clinical outcomes. Yet because of the nature of the technique, a multilevel posterior approach needs longer incision, longer muscle denervation, longer destruction on the posterior bone structure for decompression, which means the minimal invasive purpose or ideal may not always be achieved.
The nature of posterior decompression itself also has some drawbacks. The necessity for laminar bone removal in both PLIF, TLIF and also in conventional wide laminectomy can cause problems. The risk of direct neural injury when the laminar roof is removed, the risk of late fibrosis around the neural structure or the iatrogenic disruption of posterior stabilization structures during directed decompression can all be a cause of problems in the future.
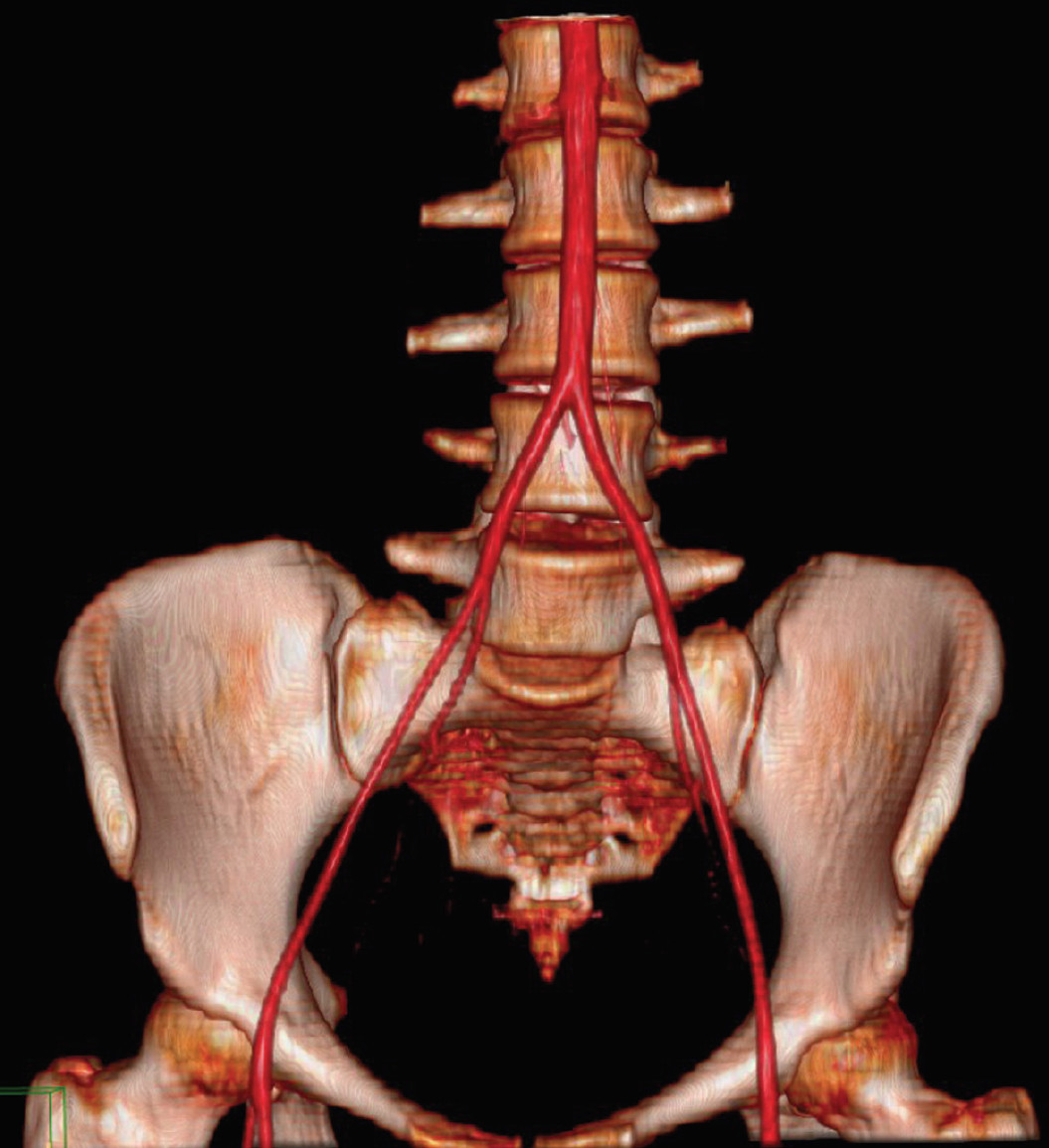
Figure 12: Show the inferiority of anterior fusion technique is the difficulty in access through the great blood vessels in front of the vertebra. The injuries of these vessel can cause catastrophic complications.
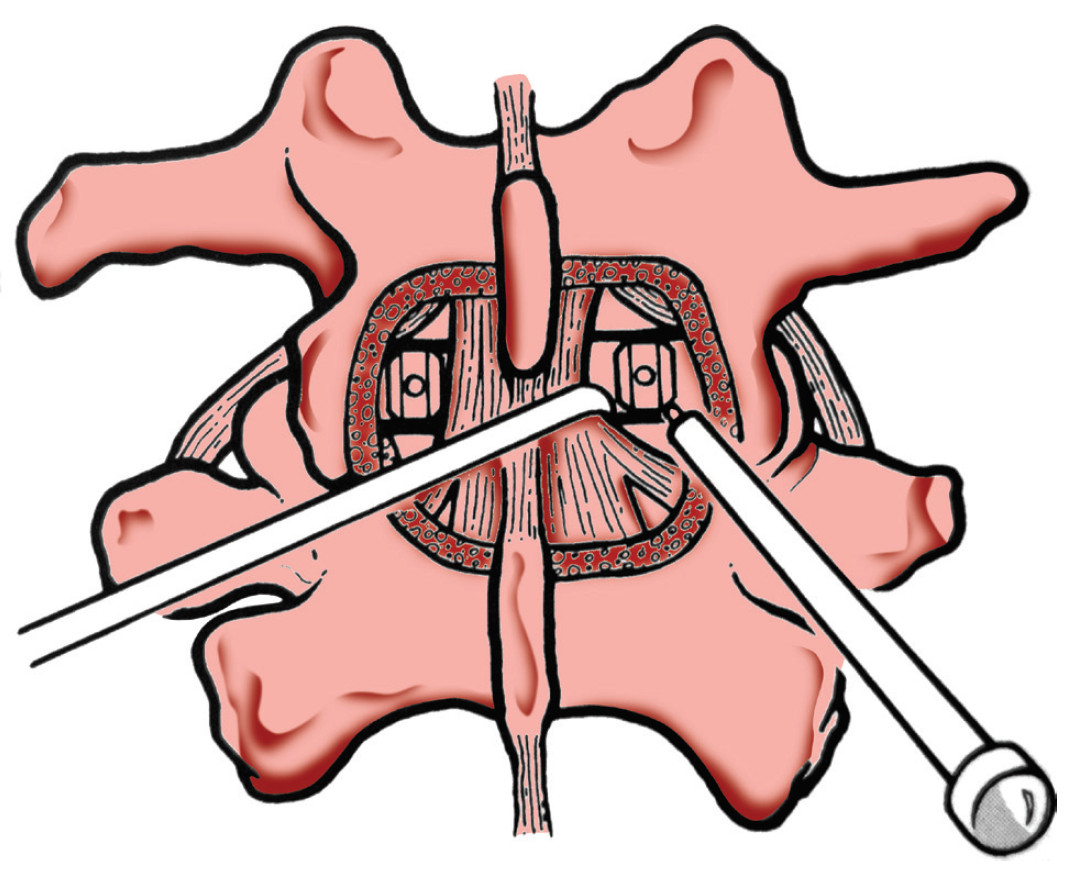
Figure 13: Show the anterior column fusion as made by posterior approach (as in the procedures “PLIF” - posterior lumbar interbody fusion and “TLIF” Transforaminal lumbar interbody fusion). Note the surgeon needs to open the spinal canal and works nearby nerve roots that can cause neural injury. Note also the small cage size inserted from the back, which needs to carry the whole body weight.
Due to the considerations mentioned above, spine specialists look forward to the ideal operation that will achieve goal of nerve decompression and best fusion results within minimally invasive strategy. The anterior column fusion has better outcomes in term of fusion rates but greater risks of serious complication. For minimally invasive anterior spinal fusion, various minimal invasive techniques have been developed such as Anterior Lumbar Interbody Fusion (ALIF) including laparoscopic, endoscopic and mini-open approaches but these all require a steep learning curve for surgeons and continue to have potential for serious complications.7
The trans-psoas lumbar interbody fusion procedure is a modification of the retroperitoneal approach to the lumbar spine using a tubular dilator/retractor system coming in the same indication of ALIF. The lateral transpsoas approach is a modification of the anterior retroperitoneal approach to the lumbar spine. The technique was first presented in 2001 by Pimenta1 in VIII Brazilian Spine Society Meeting. After that, several reports have detailed the technique, the safety of the approach, and the clinical benefits
Figure 14: Shows MIS TLIF Minimal invasive decompression and interbody posterior fusion. Even though soft tissue is protected by the tubular retractor for the minimal invasive means but the posterior inter vertebral cage is smaller size when compare to DLIF and ALIF, so the technique is inferior in terms of biomechaics and fusion rates
Figure 15: Shows MIS DLIF Minimal invasive interbody fusion from lateral approach. The portal is made by slightly smaller size tube that passes to the retroperitoneal space. It can provide space enough for placing a bigger cage inside when compared to MIS TLIF.
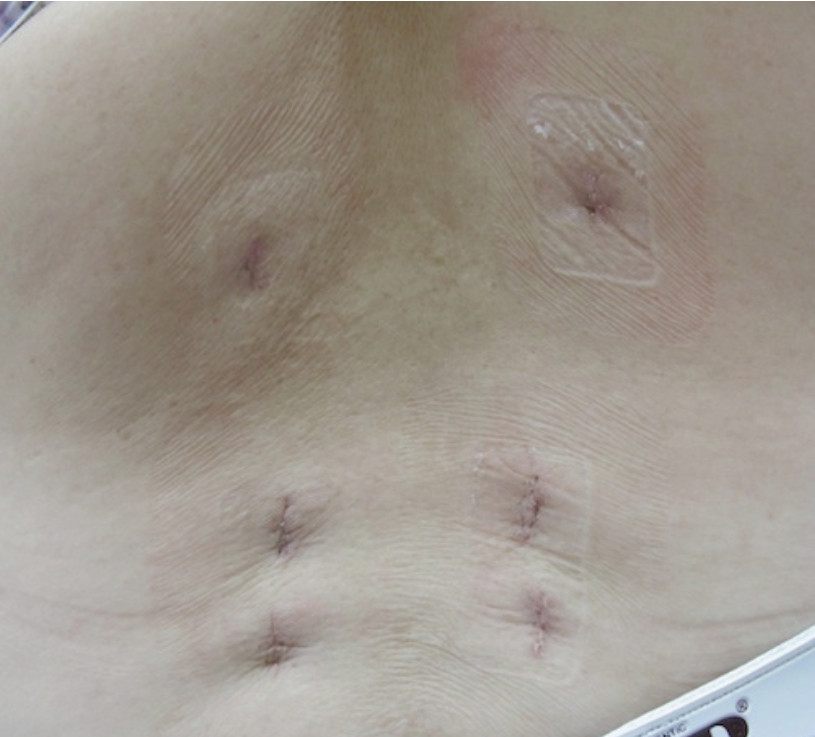
Figure 16: Shows the posterior wound scars after 1 level DLIF operation and 4-percutaneous screws fixation. Note the wound is much smaller than conventional posterior opened fusion surgery. That means DLIF has less pain, faster recovery and this technique can provide better biomechanics and fusion rates.
The advantages include elimination of great vessel mobilization thus reduction in the risk of vascular injuries and elimination of the risk of superior hypogastric plexus injuries that may cause retrograde ejaculation. Wright28 reported the outcomes of 145 cases in the United States; only 5 cases had transient hip flexor weakness. No visceral or vascular injuries and mean blood loss was 88 cc. Most of the literature reports minimal blood loss, less muscle and soft tissue destruction leading to early return to normal ability. The anterior larger cage has a big surface on which to create a better fusion when compared to posterior anterior fusion back (TLIF-PLIF). The transpsoas lumbar interbody fusion procedure is particularly useful in degenerative scoliosis condition. The large DLIF cage can reduce the coronal deformity in scoliosis patient by ensuring full bilateral end plate coverage by the DLIF cage. The minimal blood loss for this surgery increases the benefit in scoliosis in elderly. Anand et al.2 reported the mean blood loss for anterior procedure within 2-8 level scoliosis correction was only 163 cc. Finally, the procedure is relatively straight forward which makes the surgeon have a shorter learning period before being able to perform it, step by step, with less variation in each patient.
Our preliminary results show the conformity of this minimal invasive procedure. Because the procedure is straightforward, our surgeons have been able to see acceptable results as reported elsewhere in the literature. Pre-operative VAS leg pain from nerve root compression has rapidly declined even during a short follow up period. Most of our patients could ambulate by post-operative day 2-3. There were no cases where we failed to reduce radicular leg pain or back pain in a short period of surgery. The most common reported complication in other series is transient hip numbness or pain, which we also found, but it is transient and resolved within a short period of follow up. Due to the minimal disruption of back muscle and elimination of destruction of posterior vertebral structure, together with the better biomechanics of anterior vertebral column support, this procedure shows out-standing benefits in multiple level corrections such as in scoliosis, over the traditional opened posterior fusion procedure.
The radiographic result in this report shows the success in correction of coronal (scoliosis) angle. Every disc space increased in disc height; i.e. increasing the diameter of the neural foramen respectively. Therefore, this procedure should be useful particularly in L1-L5 foraminal narrowing, which is one of the pathological patterns that is difficult to solve by traditional posterior approach, especially at higher lumbar levels. The reduction in slipped distance (degree of slip) may come from the ligamentotaxis property of intact anterior and posterior longitudinal ligament in that disc level, resulting in a widening of the central spinal canal of that slipped vertebrae, without the need of direct removal of laminar and posterior ligament. That is why this procedure is also indicated in the patient with spondylolisthesis which is not more than grade 2 slipped.
Limitations of this preliminary report include the heterogeneity of our patient population. This study has a mix of patient demographics, pathologies. Scoliosis cases may not be expected to have the same results as spondylolisthesis or DDD. However, the average results demonstrate that good outcomes may still be generalized over a wide variety of patients. The one interesting indicator that we are following but cannot yet report in this study is the Oswestry disability score at pre-operative, 3 month, 6 month and at 1 year and the success of fusion rate because of the short time follow up. That data result will be very interesting and will be reported in the near future.
This preliminary short term report of DLIF at Bangkok Spine Academy shows satisfactory consistent results as other mini-opened transposes approaches. The benefits of this procedure are reducing leg and back pain, less blood loss, minimized soft tissue injury, wide safety margin of inadvertent complication especially nerve root injuries, less likelihood for long term complications and for lumbar degenerative spine in cases of decompression and fusion.
This procedure has a lot of potential to be more widely used in the near future.