Straight back syndrome (SBS) is a congenital disease of the thoracic spine and was described in 1960 by Dr Rawlings as a new cause of pseudo-heart disease as mentioned above. The pathology of SBS is defined as the loss of normal kyphosis of the thoracic spine that causes an abnormal straightness of the upper back. The thoracic spine in SBS is more straight than normal but is not stiff and can function in flexing and extending normally.2,3 The etiology of the SBS is the congenital malformation of the osseous without intrinsic bone disease.4 It always occurs in young slim patients, however, the actual level of incidence is unknown.5 It has been reported that SBS mimics some cardiac diseases that produce an abnormal cardiac murmur such as atrial septal defect, pulmonic stenosis or mitral valve prolapsed.6 This issue is the important clue to raise the awareness of SBS and to take into account that a differential diagnosis is necessary in some murmurproducing cardiac diseases.
The hallmark of the Straight Back Syndrome is the loss of normal kyphosis of the thoracic spine.5 Normal kyphosis refers to the normal apical-dorsal sagittal contour of the thoracic and sacral spine. Although the straight thoracic spine in SBS has normal spine function it behaves like a hard wall and causes a fixed narrowing of the thoracic cavity in the antero-posterior (A-P) direction. The A-P dimension of the thoracic cage in SBS is significantly shorter than normal. The fixed narrowing of the thoracic cavity in the SBS produces a fixed compression to the heart and great vessels that makes the heart and great vessels displace more to the left side of the thoracic cavity and appear enlarged. The SBS-affected patient often presents with an abnormal systolic murmur that is postulated to be due to the running of a large volume of blood flow through the fixed dilated lumen of the great vessel or may be due to the regurgitation flow that runs across the prolapsed atrio-ventricular valve back to the upstream chamber. The prolapsed atrioventricular valve in SBS may be caused by the displacement of the cardiac valve leaflets due to the fixed compression of the wall of the sternum in the anterior and from the straight thoracic spine in the posterior or this may be a coincidence. The mitral valve prolapse (MVP) is the most common associated cardiac condition reported about 64% of SBS cases.7 Contrary to this, the occurrence of SBS is not found to increase in MVP cases. On the other hand, SBS and MVP can occur in the same patient without association.8 The true congenital heart disease such as bicuspid aortic valve is also observed in the SBS patient but it is rare.2 SBS affects not only the cardiovascular system but also the respiratory system by causing compression to the trachea.7 For SBS patients, specific treatment is not required although it can become complicated with mitral valve prolapse.9
The key elements to diagnose SBS are the loss of normal kyphosis of thoracic spine that causes the narrowing of the thoracic cage in antero-posterior dimension without the other causes of bone deformity such as pectus excavatum. The pectus excavatum that produces an abnormal narrowing of the thoracic cage in the antero-posterior dimension must be ruled out before diagnosing SBS. There are proposals to establish diagnostic imaging criteria for SBS as follows:3,6,10 the measured distance from the mid anterior surface of the T8 spine to the posterior aspect of the sternum is less than 13 cm in male and less than 11 cm in female;10 the measured distance from the mid anterior surface of the T8 spine to the vertical line that connects between the top of anterior border of the T4 spine to the anterior surface of the inferior part of the T12 spine less than 1.2 cm;6 or the ratio of the distance from the mid anterior border of the T8 spine to the sternum to the length of the thoracic cage in right-left direction at the level of the dome of the right diaphragm less than 1/3.6 These criteria can be applied to the results of any imaging tools that provide the images needed for SBS diagnosis.
Conventional x-ray is the most convenient diagnostic tool for SBS diagnosis as it displays the thoracic cavity and the thoracic spine in the true coronal and true sagittal view. Multi-Detector Computed Tomography and Magnetic Resonance Imaging (MRI) may be used as optional techniques. Other investigations such as electrocardio- graphy, echocardiography may be used as supporting tools for SBS assessment. The manifestation of twelve lead ECG in cases of SBS is usually in a normal pattern or may show an incomplete right bundle branch block.6 Although twelve-lead ECG and echocardiography do not produce the typical manifestations of SBS they can be used to verify and confirm diagnosis.5
SBS must be differentiated from any causes of the abnormal narrowing of the thoracic cage such as pectus excavatum. Pectus excavatum means hollowed chest and it produces a sunken appearance of the chest.11 Although pectus excavatum produces an abnormal narrowing of the thoracic cage in the anterior-posterior direction as does SBS, Pectus excavatum can be differentiated from the SBS by the presence of the sunken sternum which is the hallmark of the pectus excavatum.11 Both SBS and pectus exacavatum affect the heart function in similar ways by causing the narrowing of the thoracic cavity in A-P dimension. On the other hand, other murmur-producing cardiac diseases are also a differential diagnosis of SBS.
MVP is the most common associated disease that has been reported. It may be caused by SBS or may be a coincidence. The differential diagnosis between MVP including other cardiac diseases and SBS must be undertaken. The key characteristics of SBS are pancake appearance with cardiomegaly; leftward heart; prominent main pulmonary artery; the AP-dimension of the thoracic cage less than 13 cm in male and less than 11 cm in female; right bundle branch block in V1 and small terminal r wave in a VR lead; and a negative echocardiography study. The characteristics of classic MVP are: the thickening of the mitral leaflet >5 mm and leaflet displacement >2 mm. The ECG sign in a classic MVP is normal or may show ST-segment and T wave abnormalities especially in lead II, III and a VF.8 If the classic signs of the mitral abnormality are found on the echocardiography or on the MRI in certain SBS cases, it should be considered that the MVP is just a coincidence.
A 25-year-old male patient, with no underlying cardiac disease, came to the hospital for an annual health check-up. An abnormal cardiac systolic ejection murmur grade II at the second left parasternal border was found during the physical examination. Chest x-rays, twelve leads ECG and echocardiography examinations were performed. The twelve leads ECG showed left axis deviation, incomplete right bundle branch block, and the trans-thoracic echocardiography showed no significant abnormalities. A cardiac MRI was requested to rule out congenital heart disease.
A cardiac MRI examination began with the scout scan using the Gradient echo MRI sequence in transverse plane with whole heart coverage that shows the mildly flat chest wall with a significant narrowing of the thoracic cavity in the A-P direction from the posterior surface of the sternum to the anterior surface of the thoracic spine. The multislice gradient echo CINE MRI was performed on the axial and horizontal planes with whole cardiac chambers coverage. The A-P dimension of each thoracic spine opposite to the entire length of sternum is measured on the axial plane and showed a maximal length of 6.43 cm. The septal leaflet of the tricuspid valve showed a mild prolapse. The gradient echo CINE MRI with short axis view of the ventricles was performed to obtain both ventricular volume and ventricular systolic function. CINE MRI on short axis view of the ventricle was performed to calculate the cardiac ventricular function using Simpson’s method. This showed a result of a normal sized left ventricle with good systolic function and mild global hypokinesia of the right ventricle. A mitral in-flow study using gradient (Q) flow mapping technique showed an E/A ratio > 2.5 with a deceleration time of 193ms that indicates impaired left ventricular diastolic function grade II. As the result of the mitral inflow study, the tricuspid inflow study also showed the E/A ratio > 2.5 that may be due to the abnormal relaxation of the right ventricle. A normal value of peak flow velocity of the aortic and pulmonic valve flow was seen. There was no abnormal thickening of atrioventricular valve. A mild septal tricuspid valve prolapse was seen on the gradient echo CINE images on the horizontal plane. The Magnetic Resonance Angiography of the aorta-pulmonary artery with contrast injection demonstrated no patent ductus arteriosus, no partial and total anomalous pulmonary venous return. A delayed contrast enhancement MRI study was also performed and the result showed no peri-myocardial contrast enhancement. This indicated no prior myocardial infarction, fibrosis and active inflammation or myocardial infiltration.
Due to the abnormal narrowing of the antero-posterior diameter of thoracic cage with pathology findings of mild tricuspid valve prolapse, SBS was suspected. The CINE MRI on the sagittal plane of the thoracic spine and the CINE MRI on the coronal plane with entire A-P dimension coverage were performed. The patient was sent to undergo a Computed Tomography (CT) scan for thoraco-lumbar spine to confirm the exact location of the T4, T8 and T12 spine. The CT image shows the abnormal downward angulation of the sternum that causes the location displacement of pairing between sternum and the thoracic spine. The widest length of the thoracic cavity of the right-left direction just at the highest point of the right dome of diaphragm was measured on MRI images including the anterior–posterior diameter from the mid anterior aspect of the eighth thoracic spine to the posterior aspect of the sternum (that is opposite to the eighth thoracic spine) were measured and found to be 5.26cm. Also, the ratio of the length of the thoracic cavity in the right to left direction to the anterior–posterior diameter of thoracic was calculated and gave a result of (5.26 cm/25.8 cm = 0.20) < 0.33. This case was diagnosed as a straight back syndrome using the criteria mentioned above.
Assessing SBS using MRI
Generally speaking, SBS is frequently diagnosed by conventional x-Rays of the chest and thoracic spine. Assessing SBS may be performed using MRI images if an MRI has been requested to rule out congenital heart disease that causes cardiac systolic murmur. An MRI has no angle limitation and has a large field of view, therefore an MRI can be used effectively to assess SBS using either Spin echo MRI or Gradient echo MRI pulse sequences. An MRI can provide a series of reference images of the thoracic spine on coronal and true sagittal views for diagnostic parameter measurements. To diagnose SBS by imaging, it is necessary to examine images of the spine on a sagittal view (this includes distal C spine, T, L, and proximal S spine) to localize the diagnostic reference point for measurement. To image the whole thoracic cavity that covers the whole length of the thoracic spine, including the width in A-P direction and the maximum length of the thoracic cavity in right-left direction, is the very least needed for SBS diagnosis and this can be obtained by MRI.
A “short-cut method” to diagnose SBS using MRI is proposed in this article. With this method, the image on the sagittal plane (with coverage of the whole length of sternum with a slice thickness of 8 mm using spin echo or gradient echo CINE MRI) is required. Then, the distance between the anterior surface of the thoracic spine to the posterior surface of the sternum is measured for each thoracic spine located between the angle of Louis and xyphoid. Normally, the heart contour occupies the space in the thoracic cavity at the level between the angle of Louis and xyphoid. According to the theory proposed by Datey and Davies, SBS can be positively identified if: the measured distance in every slice is less than 12 cm in male and 11 cm in female or the maximum length is less than 1.2 cm from the posterior surface of the thoracic spine to the vertical line connecting from the top of the anterior surface of the superior thoracic spine (that is opposite to the suprasternal notch and the antero-inferior surface of the thoracic spine that is opposite the xyphoid). Using this short-cut method for diagnosis, it is not essential to exactly localize the T4, T8 and T12 spine. It is enough to perform an MRI of the thoracic cage in the sagittal plane (covering the whole sternum and the whole thoracic spine) to diagnose the SBS. These two short-cut methods can be used together for consistency and to confirm the result. In addition, an MRI with a gradient echo CINE pulse sequence can provide information on the intra-cardiac disease that is associated with or caused by SBS. This includes the prolapsed mitral and tricuspid valve including cardiac function assessment. However, the MRI takes longer to scan compared with x-rays and CT scans of the thoracic spine.
Straight Back Syndrome causes a narrowing of the thoracic cavity in an anterior–posterior direction through loss of normal kyphosis of the thoracic spine. Although straight back syndrome is not a life-threatening disease, it should not be overlooked. It should be taken into account when the patient presents with an abnormal cardiac systolic murmur with no definite finding of cardiac cause. MRI can be considered as the one-stop-shop diagnostic tool for straight back syndrome because MRI can provide intra-cardiac views and great vessel information including the thoracic spine alignment and the diameter of the thoracic cavity in the right-left and anterior-posterior direction in a single procedure. Both spin echo black blood and gradient echo CINE MRI on sagittal view images can be used to measure the diagnostic parameters. The advantages of MRI include: providing high resolution images, a large field of view, and no angle limitation, and no radiation exposure. With MRI, congenital heart disease and other structural heart diseases can be ruled out and straight back syndrome can be diagnosed with much more confidence. In the case demonstrated in this article, the downward angulation of the sternum is observed on the MRI image when compared to the CT for the thoraco-lumbar spine. It causes the mal-position of the thoracic spine, the T4, T5 spine are not opposite to the angle of Louis and the T8, T9 spines are not opposite to the xyphoid. Hence it can be difficult to localize the reference T4, T8, T12 spine. Using the short-cut methods as described above proved to be a convenient way to diagnose SBS using MRI.
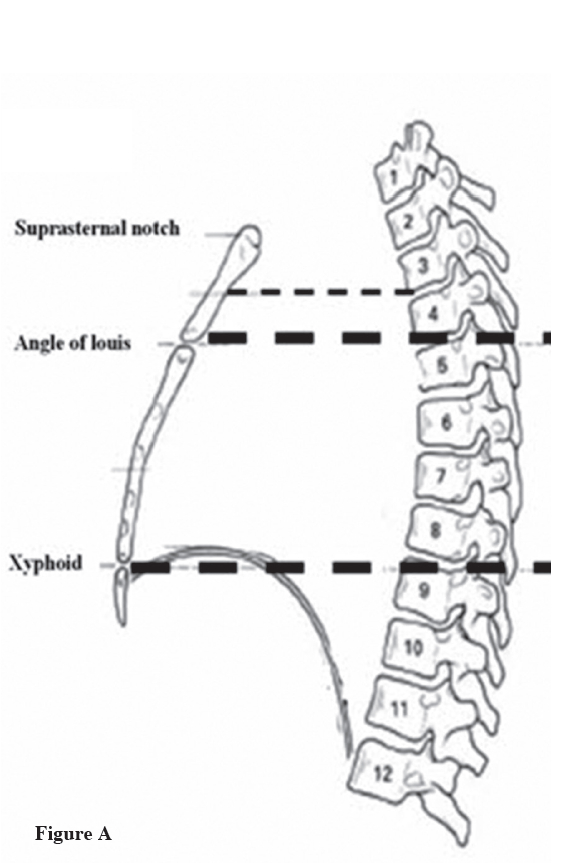
Figure A: Normal anatomy of thoracic spine and sternum. The picture shows the reference point of the thoracic spine and sternum; the suprasternal notch is situated opposite the 3rd and 4th thoracic vertebrae, the angle of Louis (manibriosternal joint) is opposite to T4 and T5 spines, the body of the sternum (the area between the angle of Louis and the Xyphoid) is placed opposite T5-T8.
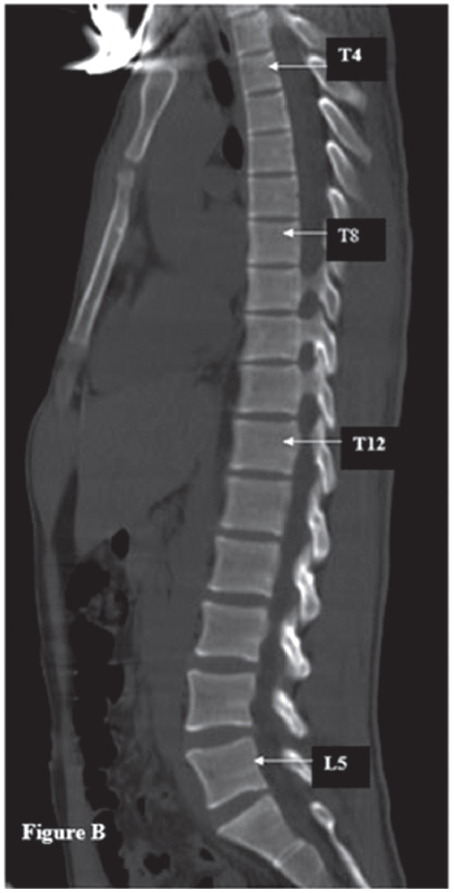
Figure B: The Computerized Tomography image on the sagittal view of the thoraco-lumbar spine of the SBS-affected patient shows the position displacement of the spine because of the loss of the normal kyphosis of the thoracic spine in SBS. The thoracic sternum is angled downwardly in the SBS hence the suprasternal notch is not situated opposite the 3rd and 4th thoracic vertebrae, the angle of Louis (manibriosternal joint) is not opposite to T4 and T5 spines, the body of the sternum (the area between the angle of Louis and the Xyphoid) is not placed opposite T5-T8.
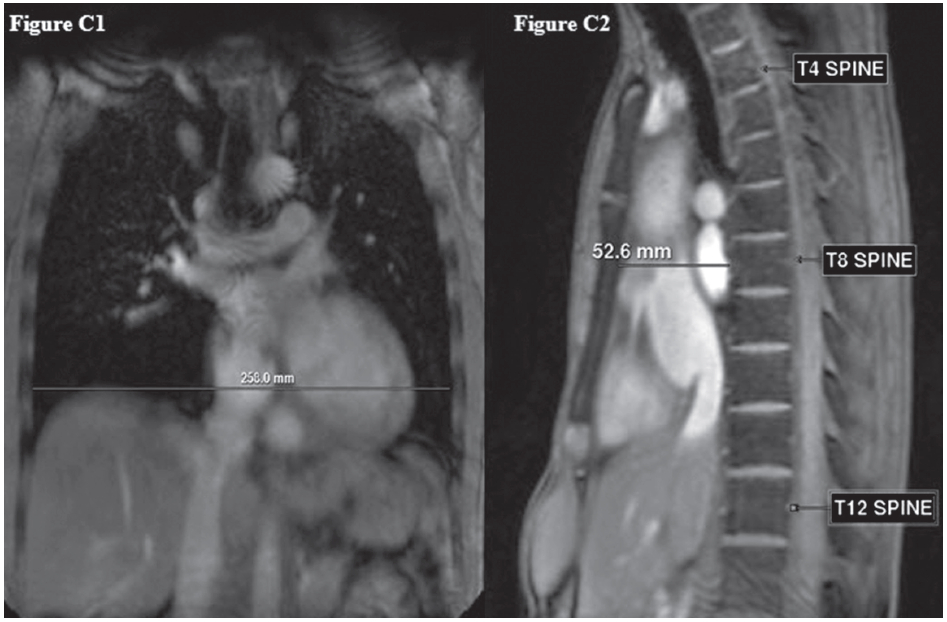
Figure C: Shows the diagnostic criteria of SBS 2; the ratio of the distance from the mid anterior border of the T8 to the sternum (C2) to the length of the thoracic cage in the right-left direction at the level of the dome of the right diaphragm (C1) less than 1/3 according to Davies’s and Leon’s proposal respectively.
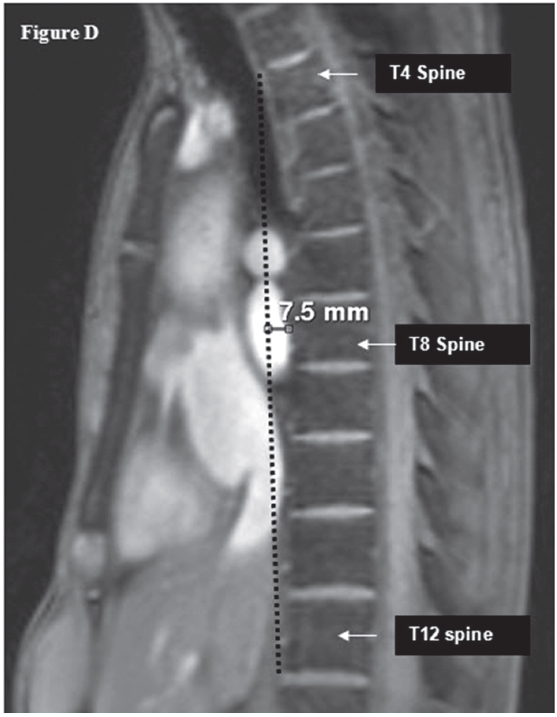
Figure D: Shows the diagnostic criteria of the SBS; the measured distance from the mid anterior surface of the T8 spine to the vertical line that connects between the top of the anterior border of the T4 spine to the anterior surface of the inferior part of the T12 spine (less than 1.2 cm).
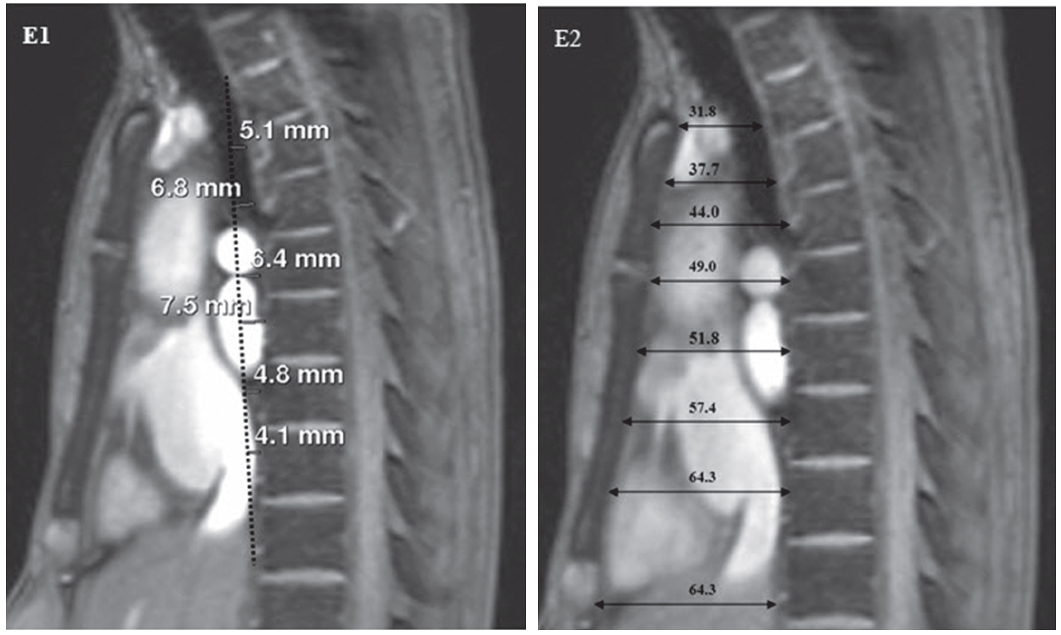
Figure E1-E2: Shows the short-cut method to diagnose SBS using MRI by measuring the distance (D2) between the anterior surface of the thoracic spine to the vertical line that connects between the posterior surface of the superior of the thoracic spine (that is opposite to the suprasternal notch and the posterior surface of the thoracic spine that is opposite to the Xyphoid) in every image slice on the sagittal view of the thoracic spine (E1). If the distance is less than 1.2 cm, SBS is diagnosed. The measurement of the A-P dimension of the thoracic cage on the sagittal view (by measuring the distance (D2) between the anterior surface of all the thoracic spine located within the length of the angle of Louis to the xyphoid to the posterior surface of sternum on the MRI image) on the sagittal view of the thoracic spine (E2) is necessary to make sure that the maximum distance value is measured. If the maximum value of the A-P dimension is less than 11 cm in female and 12 cm in male then SBS is diagnosed. The patient has D1 = 7.5 mm and D2 = 6.43 cm hence SBS diagnosis is confirmed.
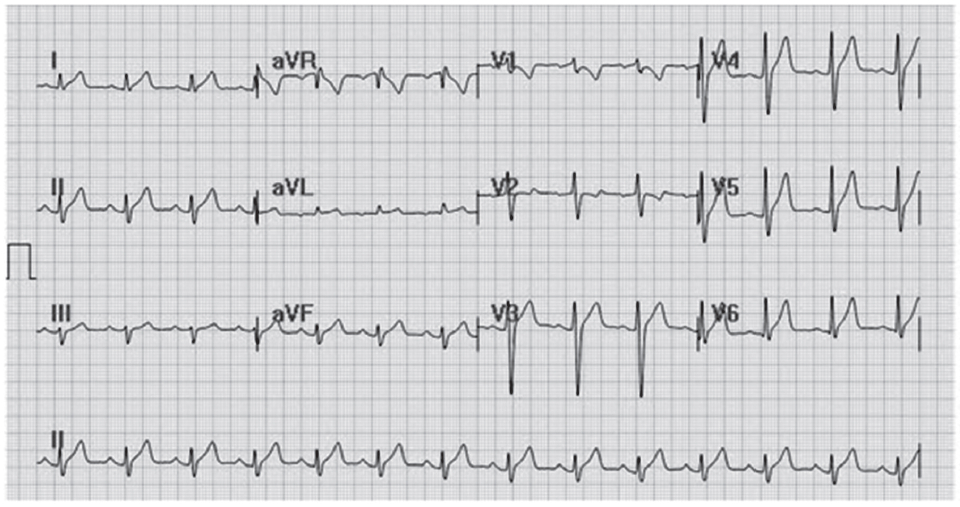
Figure F: Demonstrates the characters of the twelve lead ECG of the patient that shows left axis deviation and incomplete right bundle branch block.
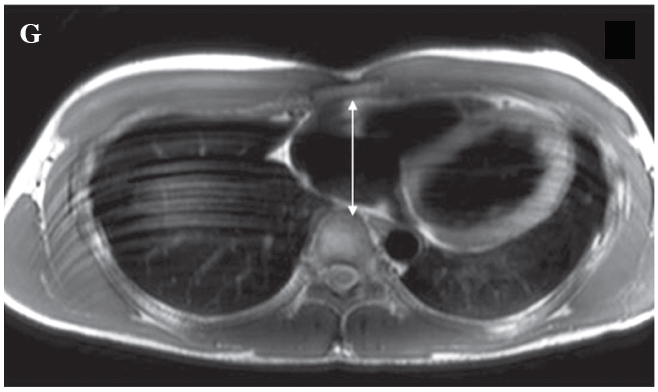
Figure G: Demonstrates the abnormal narrowing of the thoracic cage in the A-P dimension of the patient on the T1W bb image on the short axis plane.
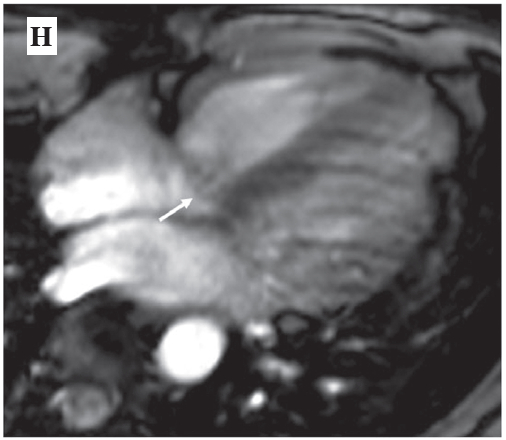
Figure H: Demonstrates the septal leaflet of the tricuspid valve prolapse of the patient (see arrow).