The history of mammography can be arbitrarily subdivided into three periods: the Age of Pioneers, the Age of Technical Progress and the Modern Era.
In 1913, Albert Salomon, a German Surgeon (Figure 1) conducted a roentgeno-histological study on over 3,000 mastectomies comparing the macroscopic observations with microscopic pathology. The first attempts to use radiography for the diagnosis of breast cancer did not become more established until four decades later.

Figure 1: Albert Salomon. (Courtesy of Andre Bruwer, MD, Tucson) Radiographic 1990;10:1111-1130
In 1930, Stafford L. Warren, a radiologist at Rochester Memorial Hospital, New York reported the use of a stereoscopic technique for in vivo mammography in 199 patients who then underwent surgery.
In 1931, Walter Vogel reported a radiographic classification of benign breast lesions and how they could be differentiated from carcinoma.
In 1938, Jacob Gershon-Cohen (Figure 2) and Albert Stricker published an article describing the range of normal radiographic of the breast.
In 1950, Gershon-Cohen emphasized the importance of high contrast images obtain without screen and with collimation and compression.

Figure 2: Jacob Gershon-Cohen (Courtesy of Radiology) Radiographic 1990; 10: 1111-1130

Figure 3: Robert L. Egan, spreading the mammography gospel, in 1967 (left) and today (right). (Courtesy of Robert L., Egan, MD, Atlanta.) Radiographic 1990;10: 1111-1130
The Modern Era
In 1960, Robert L. Egan (Figure 3) described the high- quality images technique of mammogram. The widespread use of mammography is primarily attributable to the work of Egan.
By the 1970s, dedicated x-ray machines for mammog- raphy were established and mammography was identified as the best technique for breast cancer screening.
Although, traditional film mammography is currently the gold standard in the detection of breast cancer, yet it still has several limitations e.g. underexposure, overexpo- sure, lost contrast and also less sensitivity in the detection of lobular cancer and ductal carcinoma in situ, resulting in additional study need and increased radiation exposure.1
Digital Mammography
In the past decade, mammography is undergoing the transition to digital detector, known as full-field digital mammography (FFDM). The first FFDM system, approved by the FDA in the U.S. in 2000, demonstrated that it is at least as good as screen-film mammography at detecting breast cancer without increasing breast radiation doses, or the number of woman recalled for further investigation. Digital mammography has been shown to be superior over screen-film mammography in younger women with dense breasts. FFDM selectively optimizes the contrast in areas of dense parenchymal from stromal tissue and has improved detection of disease. Technical advances with digitally stored images allow better storage, access and retrieval data. Advances in digital systems also allow better management post-image capture to produce optimal images.
Potential Harms. The possibility of false-positive test results is similar for film and digital mammography. It is uncertain whether overdiagnosis occurs more with digital mammography than with film mammography.
Costs: Digital mammography is more expensive than film mammography.2
Mammography is an effective imaging tool for detecting breast cancer at an early stage and is the only screening modality proved to reduce mortality from breast cancer. However, the overlap of tissues depicted on mammograms may create significant obstacles to the detection and diagnosis of abnormalities. Diagnostic testing initiated because of a questionable result at screening mammography frequently causes patients unnecessary anxiety and increased medial costs. Therefore, additional diagnostic exams are often required to find the right answers.
MRI is an additional investigative tool to be used after initial mammography, to detect anatomic abnor- malities, staging the local tumor and development of new blood vessels, which occur with the development of cancers. MRI is extremely sensitive and detects unex- pected disease in up to 25% of patients, which obviously has implications for their treatment. It can also detect unsuspected cancer in the other breastof some patients.
The indications for and use of breast magnetic reso- nance (MR) imaging has increased over the past decade. Current potential indications for contrast materialen- hanced breast MR imaging include (a) evaluation of the extent of known breast cancer in woman who desire breast conservation, (b) detection of contralateral breast cancer in woman with newly diagnosed breast cancer,(c) screening of women who are at high risk for devel- oping breast cancer (e.g., those with the BRCA1 gene),(d) assessment of responses to neoadjuvant therapy, (e) evaluation of chest wall invasion in patient with posterior carcinomas, (f) detection of breast cancer in woman with axillary metastasis and normal mammographic findings,3 and (g) examining breasts that contain implants, exam- ining the breasts of patients with histologically proved metastatic breast cancer with unknown primary origin.4
The sensitivity of MR imaging for the detection of breast cancer is very high, with 90% being the value reported in most studies.5, 6 However, with regard to the detection of ductal carcinoma in situ (DCIS), the sensi- tivity of MR imaging varies between 40% and 100%.7 The result may be false-negative in the presence of DCIS or an invasive ductal or lobular malignancy. Specificity of 37%-100% has been reported for breast MR imag- ing; in most studies, it has varied from 50%-70%.5 The relative low specificity of breast MR imaging is a disadvantage, and rigorous criteria have been proposed for the interpretation of breast imaging.8
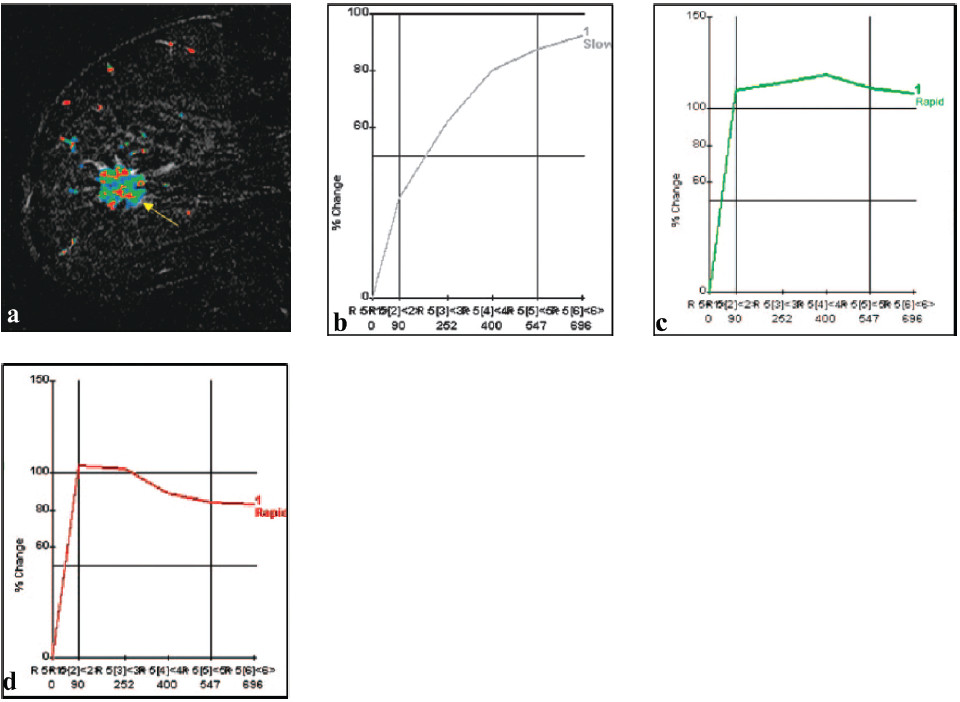
Figure 4: Tumor heterogeneity in a 69-year-old patient with grade I and III invasive ductal carninoma. (a) Sagittal postcontrast T1-weighted subtraction image shows heterogeneous tumor enhancement (arrow). (b-d) Computergenerated kinetic curves obtained within the lesion show the three classic types of kinetic assessment curves: the slow persistent curve (type I) (b), plateau curve (type II) (c), and wash-out curve (type III) (d). (RadioGraphic October 2007; 27)
The specificity of breast MR imaging is improved when both morphologic and kinetic features are consid- ered in the interpretation. In classifying breast lesions, the assessment of margin and qualitative enhancement intensity (at 2 minutes or less after contrast material injection) is the most important features currently avail- able for breast mass characterization. The next most important feature is the qualitative assessment of the enhancement kinetic curve.
Kuhl and colleagues9 have described three time-signal intensity curves that are important in differentiating benign from malignant lesions. The type I curve (Figure 4b) is a slow steady enhancement curve. The type II curve demonstrates plateau signal intensity (Figure 4c). The type III curve is associated with washout of signal intensity (Figure 4d) andis strong indicator of malignancy. The use of time-signal intensity curves has a sensitivity of 91% and specificity of 83%.9
False Positive
• Overlap of benign and malignant lesion
• Incidental enhancing lesion, common in premenopausal women with dense breasts
False Negative
• Invasive lobular carcinoma
• Low grade ductal carcinoma i.e. tubular DCIS
Potential Harm: Contrast-enhanced MRI requires the injection of contrast media. Studies of MRI screen- ing have shown that MRI yield many more falsepositive results than does mammography. MRI has potential to be overdiagnosis than mammography.
Costs: MRI is much more expensive than either film or digital mammography.
Current Practice: MRI is not currently used for screening women at average risk of breast cancer because of its being less specific than mammography screening. There is the potential, it could be be associated with higher biopsy rates and a greater degree of overdi- agnosis if used in low-risk populations.
Dual Energy Contrast Enhanced Digital Mammography(DE CEDM)
The accuracy of conventional mammography is limited in dense breasts where surrounding fibroglandular tissue decreases the conspicuity of lesions and lack of intrinsic tissue contrast. Even when tumors are detected, the full extent of disease may not be clearly depicted. The growth and metastatic potential of tumor can be directly linked to angiogenesis. Tumor angiogenesis factors stimulate formation of abnormal vessels that leak and shunt blood. Therefore, imaging methods with contrast medium potentially can aid in the detection and diagnosis of cancer.10
In the mid-1980s, Watt et al.12, Ackerma et al.13, and Watt et al.14 performed digital subtraction angiography (DSA) of the breast by using and x-ray imaging intensi- fier system. Benign and malignant lesions were differen- tiated according to the strength of enhancement.
The technique consists of high and low energy digital mammography after administration of iodinated contrast agent. DE CEDM is similar in concept to enhanced breast MRI imaging in detected new blood vessels. It can be used to detect primary breast cancer in a woman with a positive axillary lymph node and determine the extent of disease in cases of known cancer, as well as problem solving in case of mammographic findings that were not depicted in additional mammograms or US scans.
Its remains to be seen whether the sensitivity to cancer is as high for DE CEDM as it is for MRI, which has been shown to have a very high sensitivity. Both techniques make use of the same property of tumor angiogenesis, which causes cancers to take up contrast agent faster and to a greater degree than do normal tissues or benign masses, because of denser capillaries that are also abnormally “leaky”. Because of its higher contrast resolution, MRI is probably more sensitive to contrast enhancement than is DE CEDM, but the degree to which that translates into higher sensitivity for cancer detection is unknown. One drawback of MRI is that its high sensitivity to contrast agent uptake causes it to be plaqued by numerous false-positive foci of enhancement. MRI also has relatively limited sensitivity to ductal carcinoma in situ (DCIS), which is depicted as microcalcifications at mammography. DE CEDM was 83% specific in this study, and the lowenergy source images showed microcalcifications, which could be used in the diagnosis of DCIS.
Findings with MRI imaging suggest that morphologic features (i.e., shape and margin) help differentiate benign from malignant enhancing areas. Because DE CEDM allows a higher spatial resolution than that with MRI, differentiation of benign from malignant morphologic features at DE CEDM should be easier. Enhancement kinetics, also used for differentiating benign from malignant lesions at MRI can be determined at DE CEDM with serial imaging. Because whole breast images can be acquired more rapidly than with most MRI sequences, kinetic formation could be determined with greater precision. Unlike MRI, however each image has a penalty of additional radiation. DE CEDM is less expensive than MRI.
The expansion of existing mammographic core biopsy or preoperative needle localization techniques to include DE CEDM would be straightforward, give the right equipment. Such procedures are difficult to perform with MRI guidance because of the geometry of a high- strength magnetic field.10
The results of the study of Roberta et al.11 suggest that contrast-enhanced digital mammography potentially may be useful in the identification of lesions in the mammographically dense breast. As in MR imaging, other applications may be in the identification of the extent of disease or in the identification of an otherwise occult carcinoma that has manifested with axillary metastases. This information may aid in the diagnosis and guidance of core-needle biopsy or excision of these lesion. Furthermore, with the increasing availability of digital mammography, contrast-enhanced digital mammography will become accessible and relatively inexpensive compared with current MR imagingtech- nology. These results encourage further investigation of contrast-enhanced digital mammography as a diagnostic tool for breast cancer.
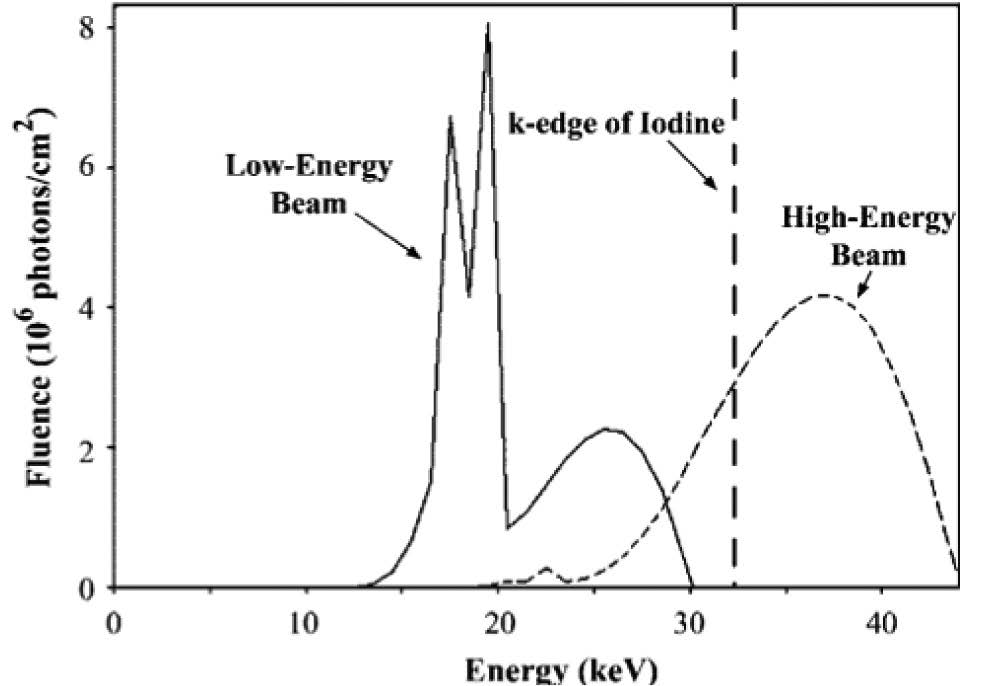
Figure 5: X-ray spectra calculated for high and lowen- ergy beams. Each curve is scaled to represent exposure through a 4.5-cm-thick 50% glandu- lar-50% fat breast. High-energy parameters include44kVp, rhodiumanode, 0.025-mm-thick rhodium and 8-mm-thick aluminum filters, and 200 mAs. Low-energy parameters include 30 kVp, molybdenum anode, 0.03-mm-thick molybdenum filter, and 140 mAs. The k edge of iodine, at 33.2 keV, is marked by a dashed line. (Modeling program courtesy of General Elec- trical Corporate Research and Development, Niskayuna, NY.) : Radiology October 2003.
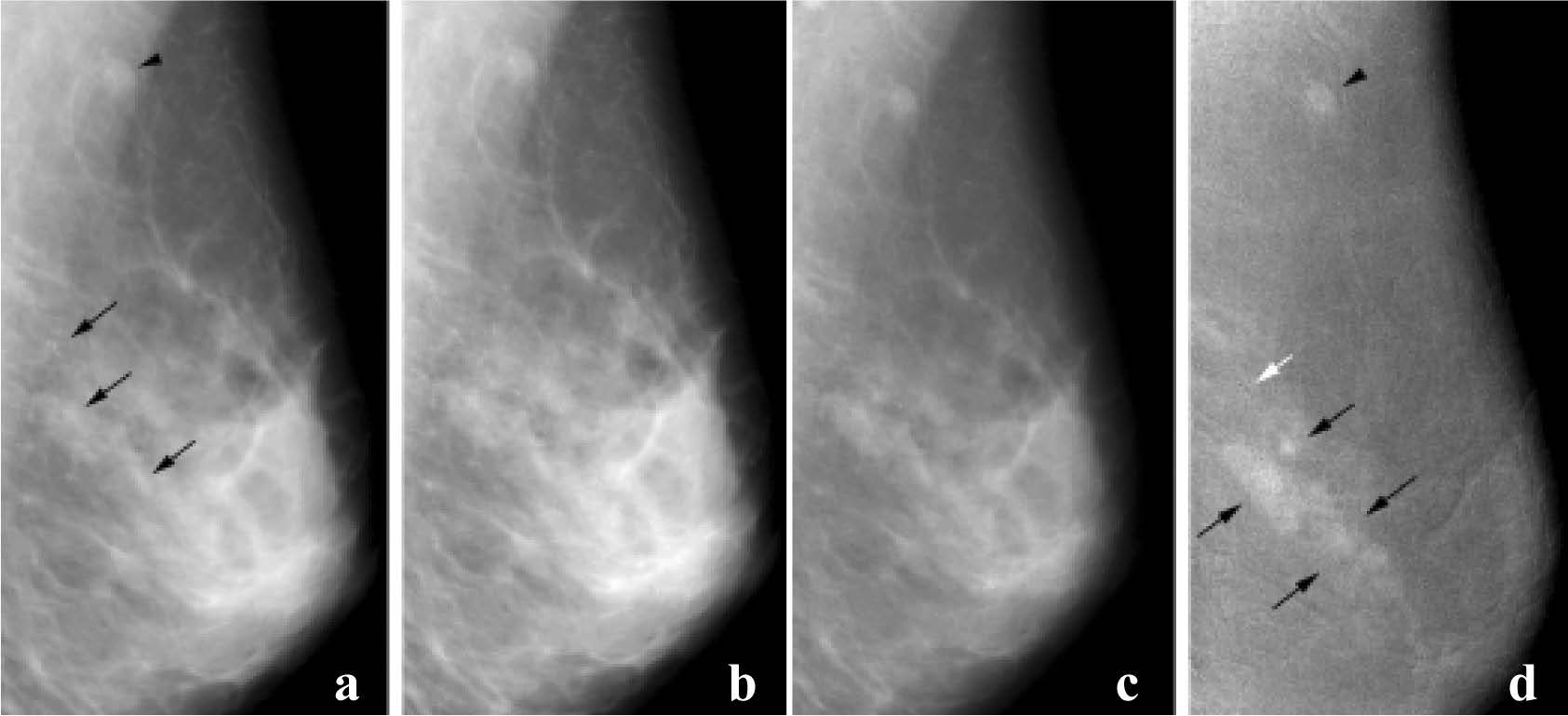
Figure 6: Invasive ductal carcinoma and DCIS. (a) Mediolateral oblique mammogram shows grouped microcalcficationin in the breast (arrows) and in lymph node (arrowhead). Enhancement is barely perceptible on postcontrast (b.) low-energy and(c) high-energy images. (d) Subtracted dual energy enhanced DSM image shows the invasive component as enhancing lesions (black arrows), but there is no definite enhancement around grouped calcifications in the posterior breast (white arrow). The malignant lymph node (arrowhead) is also enhanced. (Radiology 2003; 229:261-268)
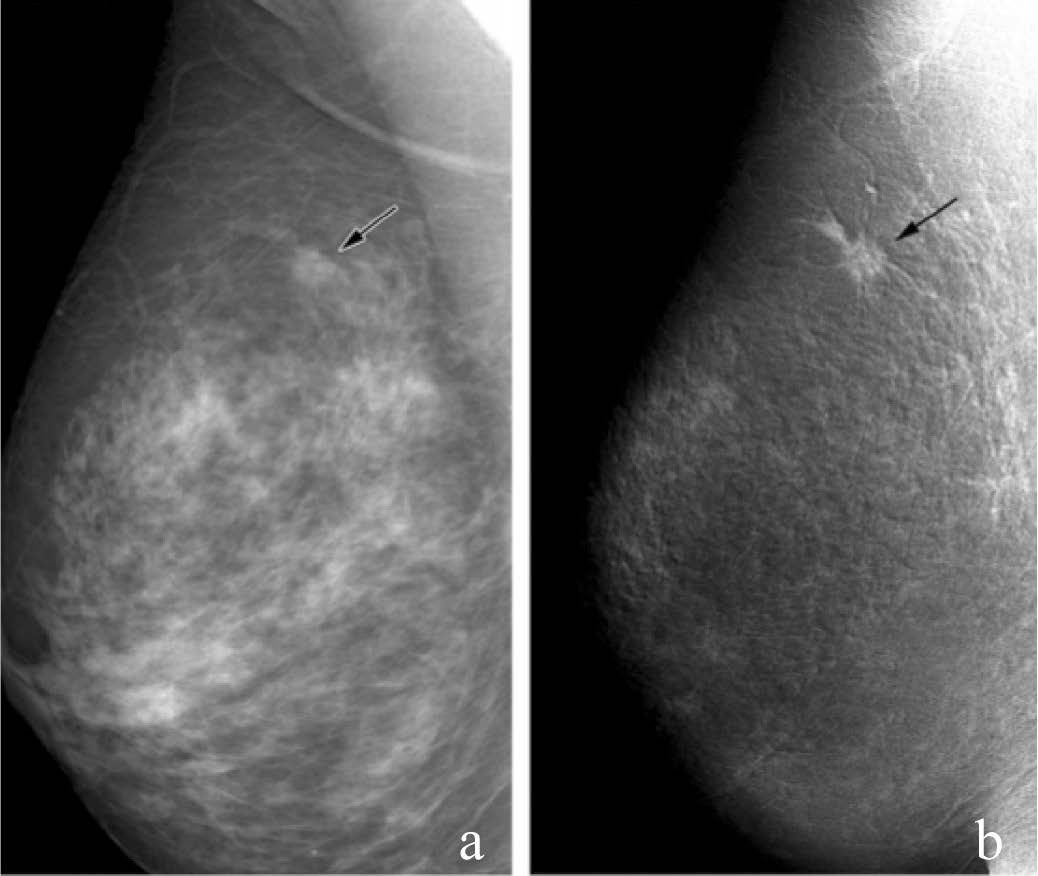
Figure 7: Invasive ductal carcinoma (11-mm diameter). (a) Mediolateral oblique mammogram shows possible spic- ulated mass (arrrow). (b) Dual- energy enhanced DSM image shows the cancer as an enhancing mass with definite spiculations (arrow). (Radiology 2003; 229:261-268)
Generally, breast cancer tissue is harder than the adjacent normal breast tissue. This property serves as the basis for some examination, such as palpation, that are currently being used in the clinical assessment of breast abnormalities, as well as for elastography.
The principle of elastography is that tissue compres- sion produces strain (displacement) within the tissue and that the strain is smaller in harder tissue than in softer tissue. Therefore, by measuring the tissue strain induced by compression, we can estimate tissue hardness, which may be useful in diagnosing breast cancer.
Ako et al.22 findings of a significant difference between mean elasticity scores for malignant and benign lesions in patients suggests that elastography may be useful in diagnosing breast lesions in the clinical setting. To classify elasticity images,they evaluated the color pattern both in the hypoechoic lesion (i.e.,the area that was hypoechoic or isoechoic relative to the subcutane- ous fat [except for echogenic halo] on Bmode images) and in the surrounding breast tissue. On the basis of the overall pattern, they assigned each image an elasticity score on a five-point scale.
Ako et al.’s22 believe that an elasticity score of 5, which shows no strain in the entire hypoechoic lesion and the surrounding are at B-mode US, indicates infiltra- tion of cancer cells into the interstitial tissue (e.g., in scir- rhous carcinoma) or into an intraductal component (e.g., in DCIS), both of which are characteristics of carcinoma.
An elasticity score of 4, which indicates no strain in the entire hypoechoic lesion, seems to be characteristic of tumors such as solid tubular carcinomas that are circumscribed and homogeneously harder than the adjacent normal breast tissue.
An elasticity score of 3 which indicates strain at the periphery of the hypoechoic lesion, was mainly found in benign lesions, including intraductal papillomas. The importance of strain at the periphery is unclear at present and requires further investigation. Ako et al.22 recom- mend that all lesion with elasticity scores of 3 or higher be examined by means of aspiration cytology or needle biopsy because two (13%) of the 15 lesions with a score of 3 were malignant.
An elasticity score of 2, for which pars of the hypoechoic lesion did not show strain at B-mode US, indicate lesions that are soft yet somewhat harder than normal breast tissue. This is often characteristic of lesions such as fibroadenoma, duct papillomatosis, sclerosing adenosis or lobular hyperplasia.
An elasticity score of 1, which shows even strain in the entire hypoechoic lesion at B-mode US, indicates that lesions have almost the same compressibility as the surrounding breast tissue. In this study22 no malignant lesions had a score of 1.
In conclusion, elastography could be complementary to conventional US, thereby making it easier to diagnose breast lesions. The skill needed to acquire adequate images is similar for conventional US and elastography; the skill needed to interpret images. Elastography is promising, and it is expected that with future improvements in the technology (e.g., the development of a pressure indicator and approaches for quantitative assessment), this imaging modality will become an invaluable tool for the diagnosis of breast disease in the clinical setting.22
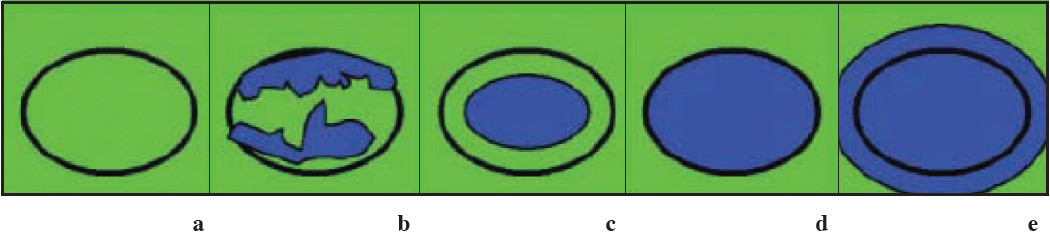

Figure 8: Images present general appearance of lesions for elasticity scores of (a)1, (b)2, (c)3, (d)4, and (e) 5. Black circle indicates outline of hypoechoic lesion (ie, border between lesion and surrounding breast tissue) on B-mode images. (Radiology 2006;239:341-350)
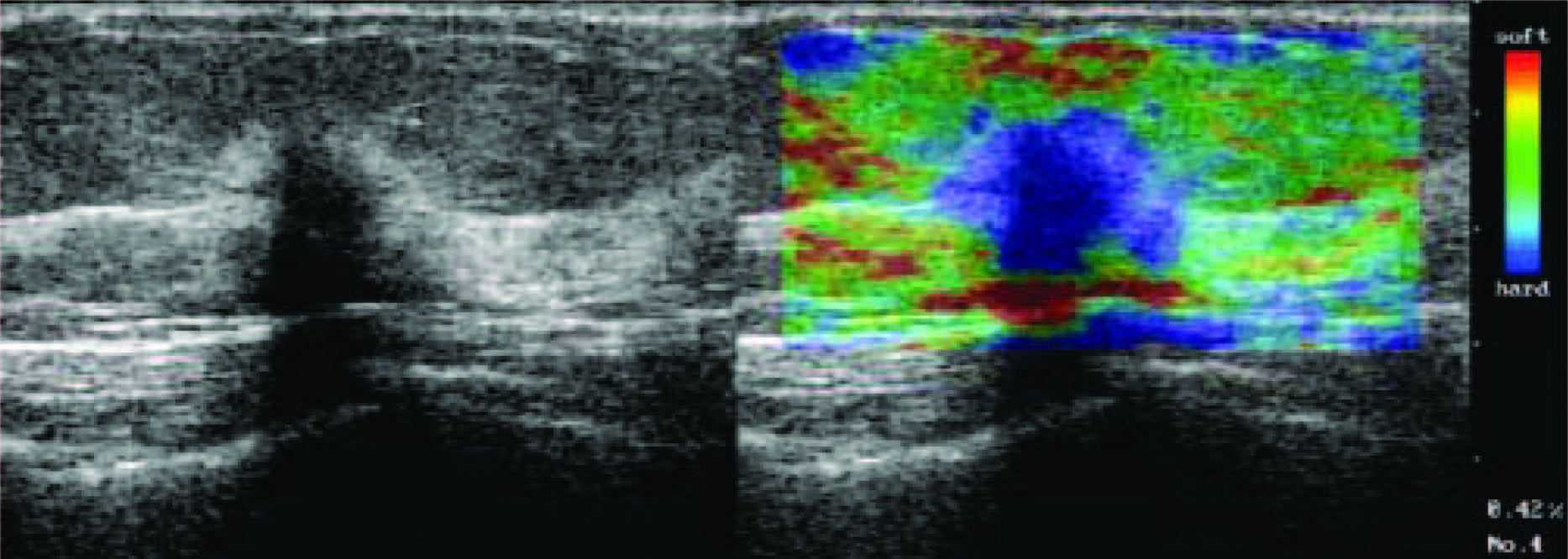

Figure 9: Scirrhous type invasive ductal carcinoma with elasticity score of 5 in 55-year-old woman. US images were obtained in sagittal plane. Left: On conventional B-mode images, lesion was classified as BI-RADS category 5. Middle: On elasticity image, both the entire hypoechoic lesion and its surrounding area were blue. (Radiology 2006;239:341-350)
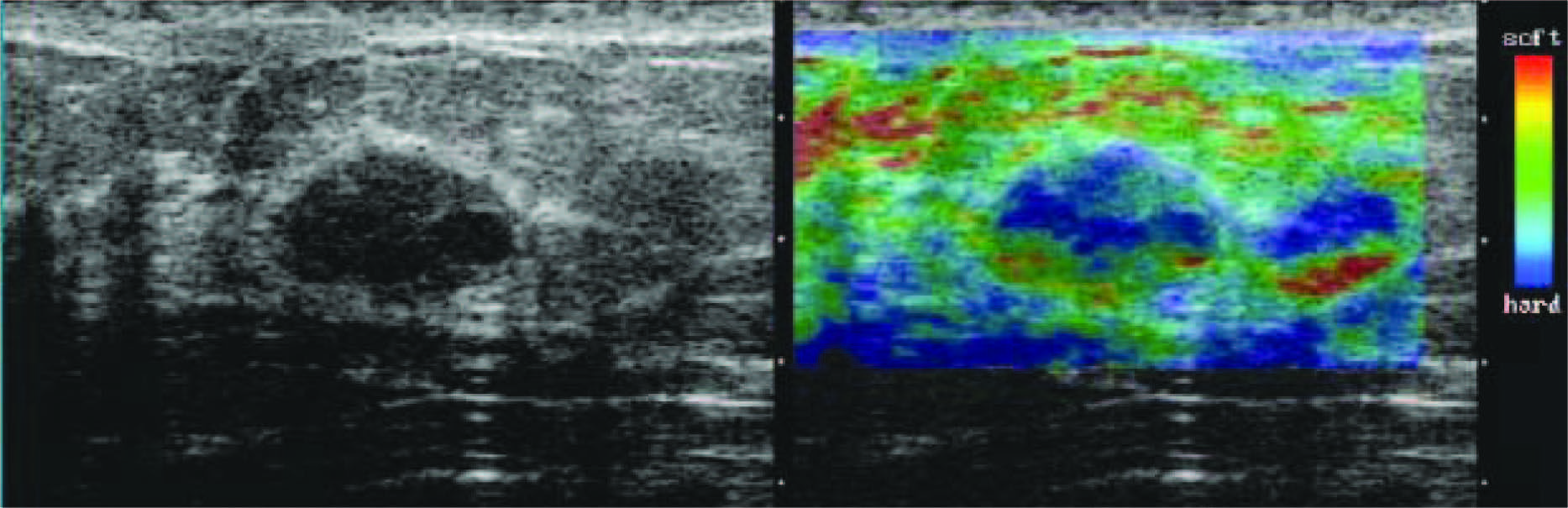

Figure 10: Fibroadenoma with elasticity score of 2 in 39-year-old woman. US images were obtained in transverse plane. Left: On conventional B-mode image, lesion was classified as BI-RADS category 3. Right: On elasticity image, hypoechoic lesion shows mosaic pattern of green and blue. (Radiology 2006;239:341-350)
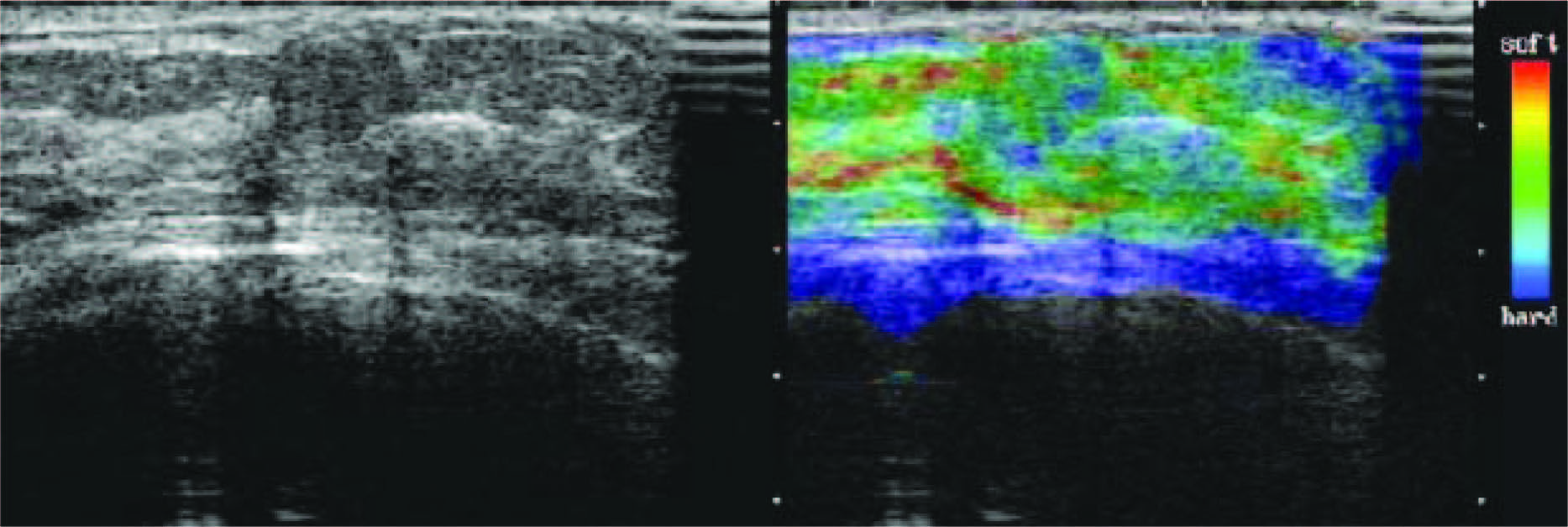

Figure 11: Fibroadenoma with elacticity score of 1 in 51-year-old woman. US images were obtained in transverse plane. Left: On conventional B-mode image, lesion was classified as BI-RADS cathegory2. Right: On elasticity image, the entire hypoechoic lesion was evenly shaded green, as was the surrounding breast tissue. (Radiology 2006;239:341-350)
Breast tomosynthesis is a new tool that can reduce or eliminate tissue overlap. Breast tomosynthesis technology is essentially a modification of a digital mammography unit to enable the acquisition of a threedimensional (3D) volume of thin section data. Images are reconstructed in conventional orientations by using reconstruction algo- rithms similar to those used in computed tomography (CT).
DBT consists of taking multiple images at multiple angles that can reduce or eliminate the tissue overlap effect that occurs so frequently with 2-D film screening mammography.
Images are reconstructed in conventional orienta- tions by using reconstruction algorithms similar to those used in CT. DBT imaging enables 3-D breast examina- tions, overlapping of tissue at interpretation of project- ing mammograms. DBT’s potential advantages include reducing lesion localization and the selection of patients for biopsy, as well as assessing the therapeutic efficacy of chemoradiotherapy. Similar technical improvements from digital imaging supplement these performance parameters, as well.
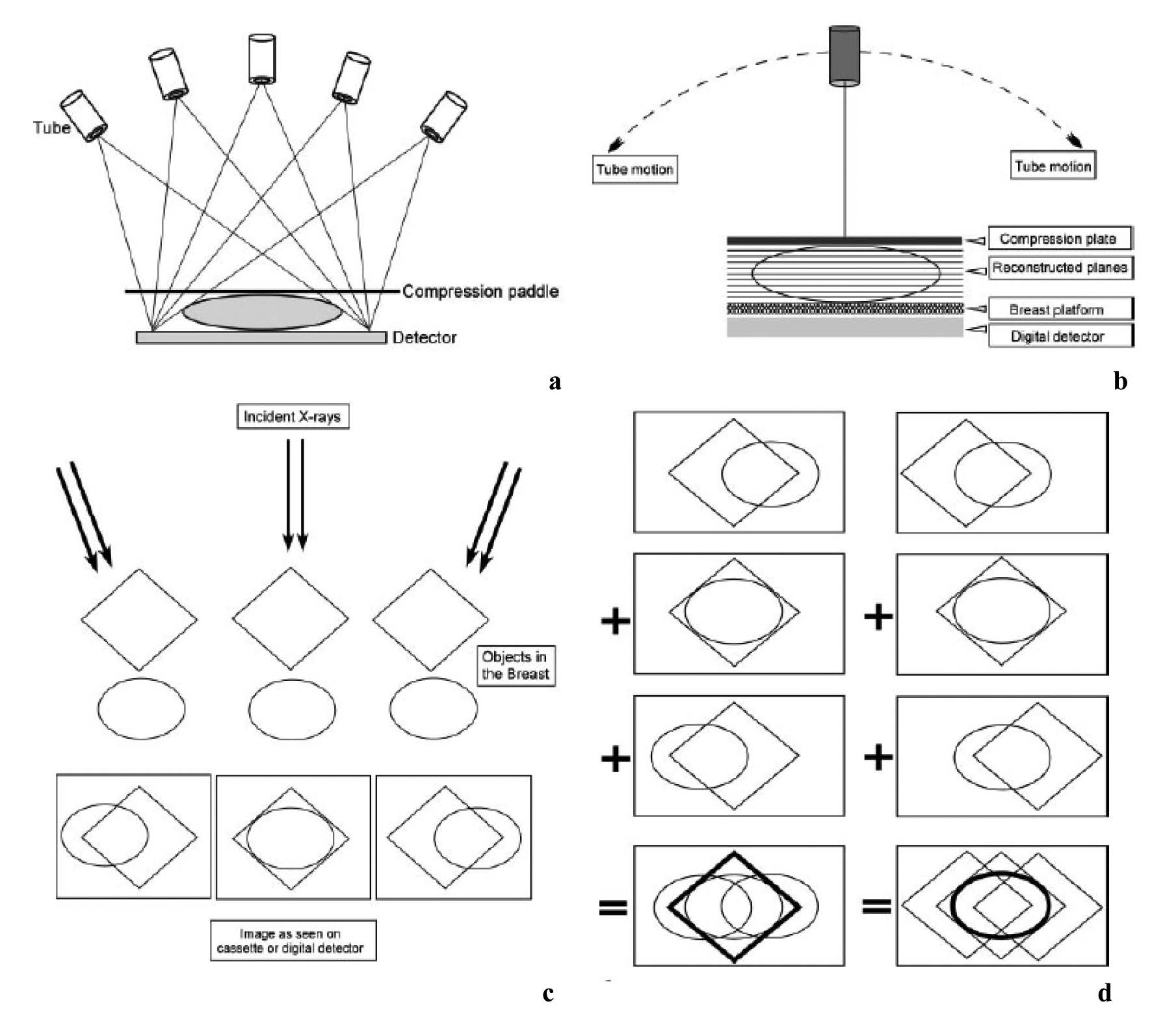

Figure 12: Basic technologic principles of breast tomosynthesis. (a,b) Schemans shows how image data are acquired from various angles as the x-ray tube moves in an arc. Either the step-and-shoot method (a) or the continuous exposure method (b) may be used, and the detector may be moving or stationary during image acquisition. The 3D image data are subsequently reconstructed as conventional mammographic projections (cranio-caudal, mediolateral oblique and mediolateral view). (c, d) Diagrams show how different 3D image data aquired from different angles (c) are reconstructed to provide separate depiction of two overlapping structures located in different planes (d). 15
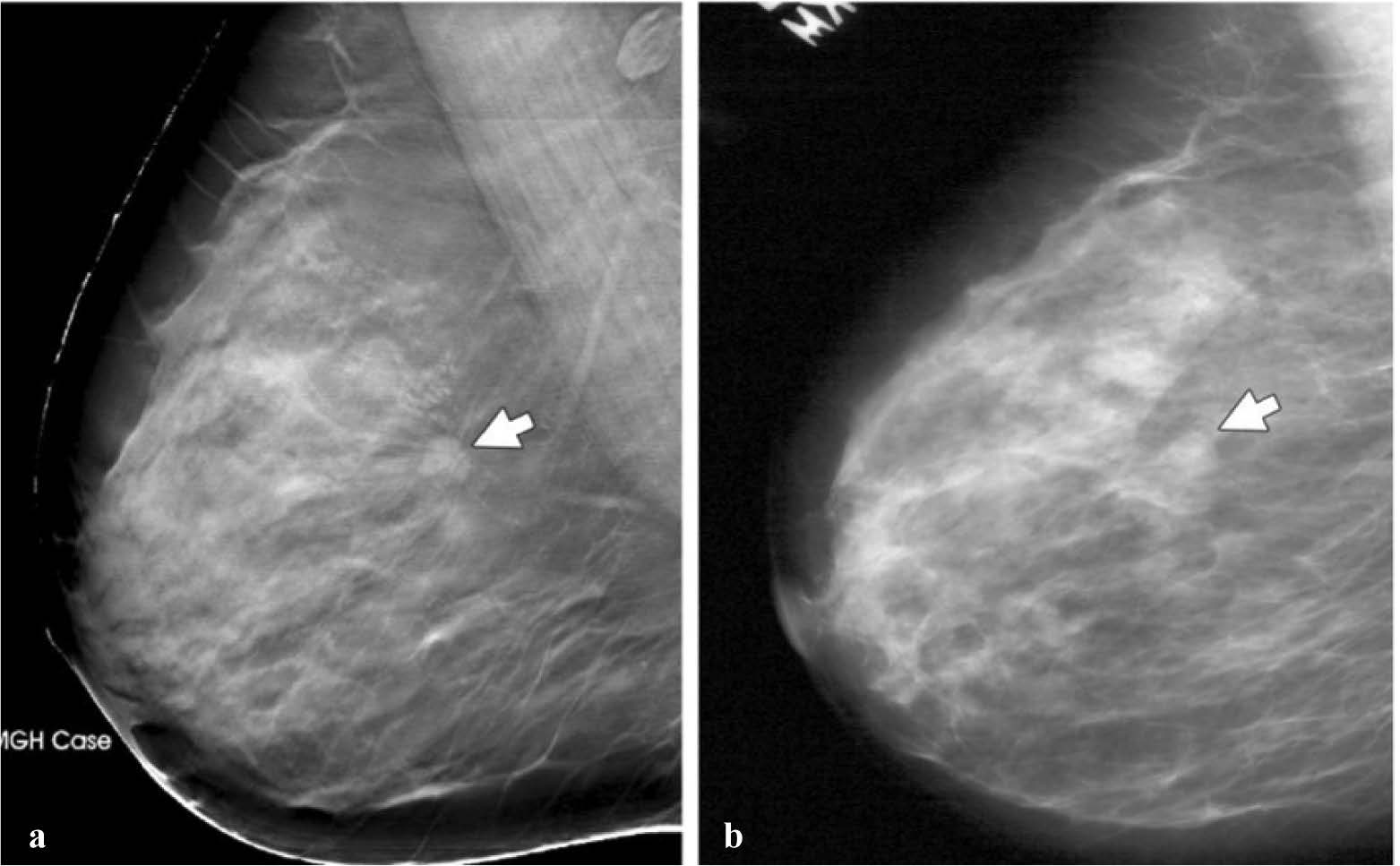

Figure 13: Mass (arrow) depicted in the mediolateral oblique view on (a) and (b) screen-film mammograms. The spicules of the mass are much more conspicuous. (Radiology 2005; 237:1075-1080)
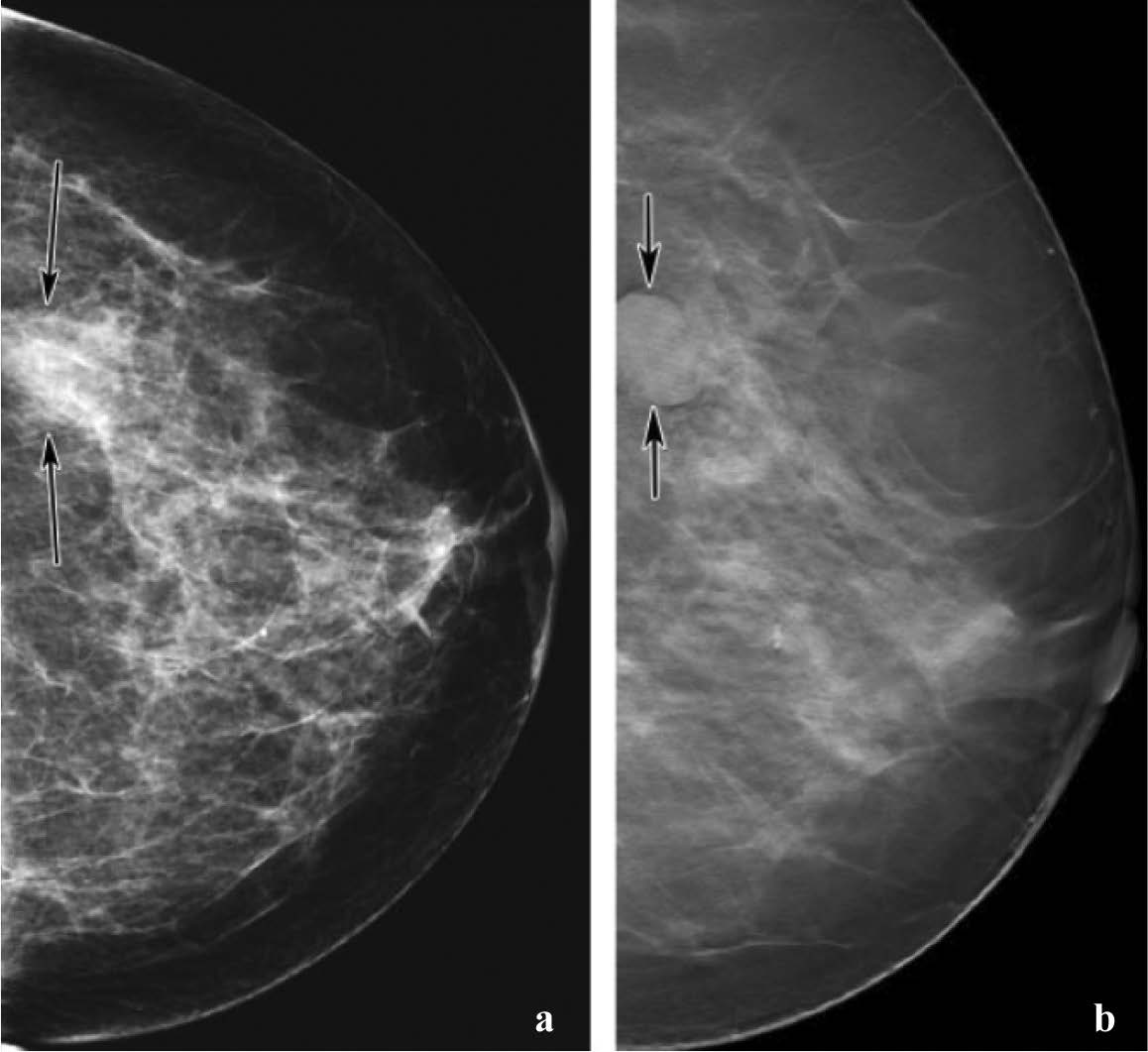
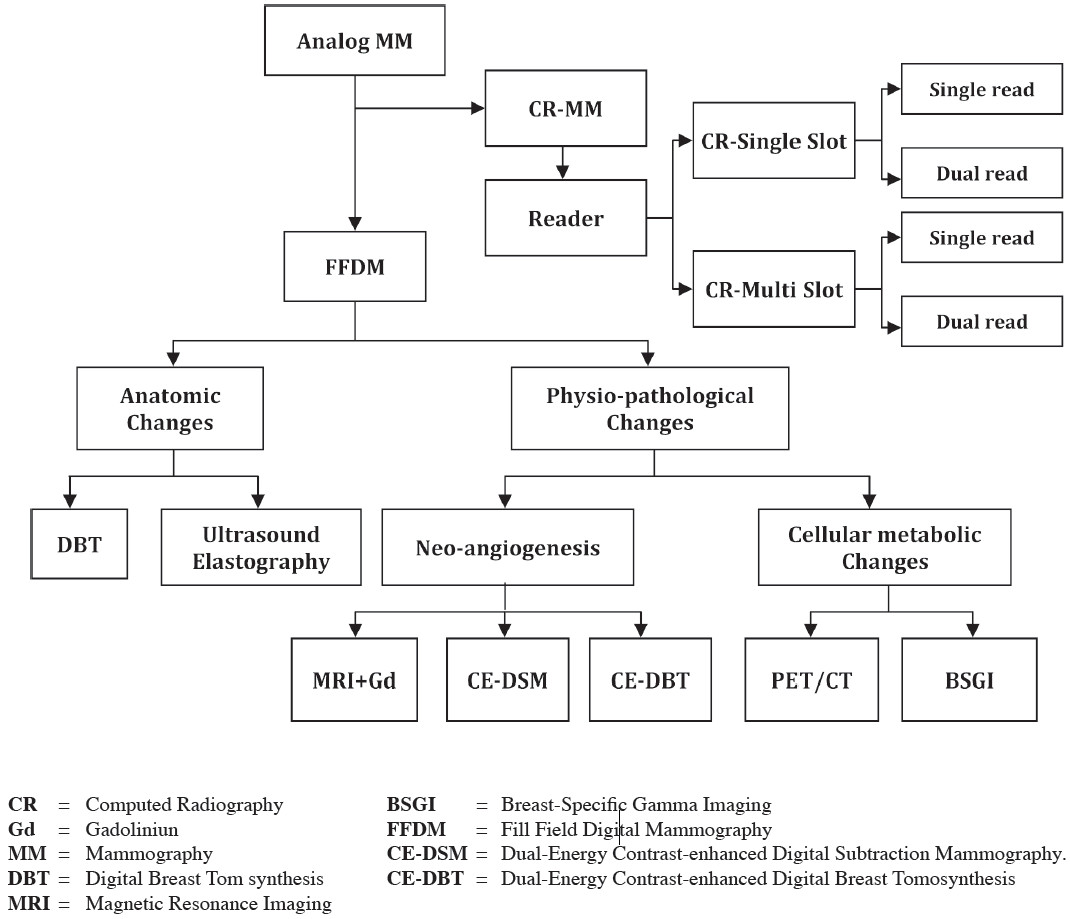
Figure 14: Comparison of screening mammography with breast tomosynthesis in a 57-year-old woman. (a) Digital mammogram shows a mass (arrows) in the lower outer part of the left breast. The mass is not clearly visible because of surrounding dense tissue. (b) Breast tomosynthesis image provides clearer depiction of the mass (arrow), which is well circumscribed. Because its’ US appearance remained stable for 2 years, the mass was considered benign.(See also Movie 1at radiographics.rsnajnls.org/cgi/content/full/27/S231/DC1) 15
CE-DBT is a combination of the projection tech- niques of DBT with intravenous contrast enhancement. As tumor angiogenesis is necessary for breast cancer growth, the use of diffusion contrast enhancement utilizes the increased vessel permeability in the disorganized vessel network to better visualized the tumor function. Lesions are identified by the use of an iodinated contrast agent, which tends to pool in the tumor space. X-ray techniques using contrast agents have demonstrated the enhancement of breast cancers and increase in lesion conspicuity. This combination of contrast-enhance digital mammography and DBT into a single technique would potentially integrate the benefits of both technique, thus providing both breast cancer morphology and vascular information. These advantages of CE-DBT will be better with conventional mammography for biopsy or preop- erative localization purposes. Furthermore, CE-DBT may be cheaper than breast MR and potentially be more widely available.
More recently, dedicated breast positron emission mammography (PEM) units have been developed to overcome the limitation of whole-body PET and to provide a positron-emitting imaging platform capable of detection and depiction of primary breast carcinoma (Figure 11).
In general, these systems consist of two planar detectors placed opposite a gently compressed breast. The advantages of such dedicated systems include improvement geometric sensitivity, higher spatial reso- lution, shorter imaging time, and reduced attenuation compared with whole-body PET systems. They also have a small physical footprint, which makes their use in a breast imaging facility feasible and allows correlation of the results with those of conventional breast imaging as well as PEM-guided biopsy.
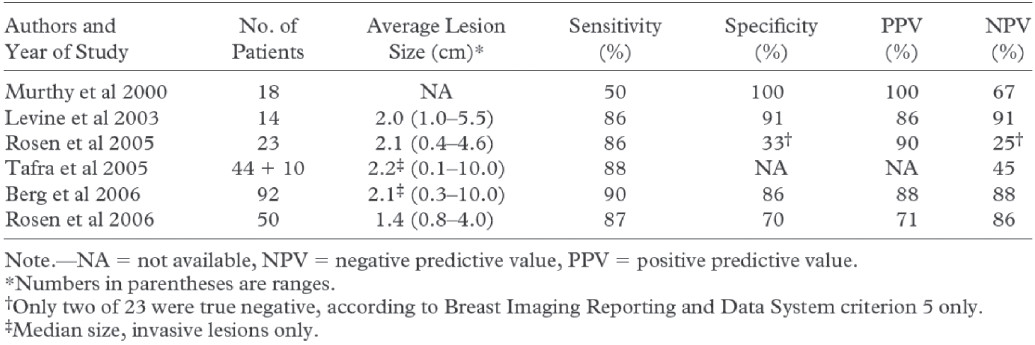
Although preliminary data demonstrate that these systems are capable of imaging smaller primary breast carcinomas than whole-body systems, their clinical utility has not been adequately demonstrated (Table 1)18-19. Certain limitation such as imaging posterior lesions, variable FDG uptake in small tumors, and falsepositive findings from prior biopsy have been reported, and biopsy capability needs to be further addressed8,10-12. Potential roles advocated for these systems included detection, problem solving, local staging, local recurrence, and assessing or predicting response of the primary tumor to chemotherapy. Dedicated breast PEM and PET are a promising technology to help overcome the limitations of whole-body imaging and may eventually provide a positron emission imaging platform capable of reliably imaging primary breast carcinoma.
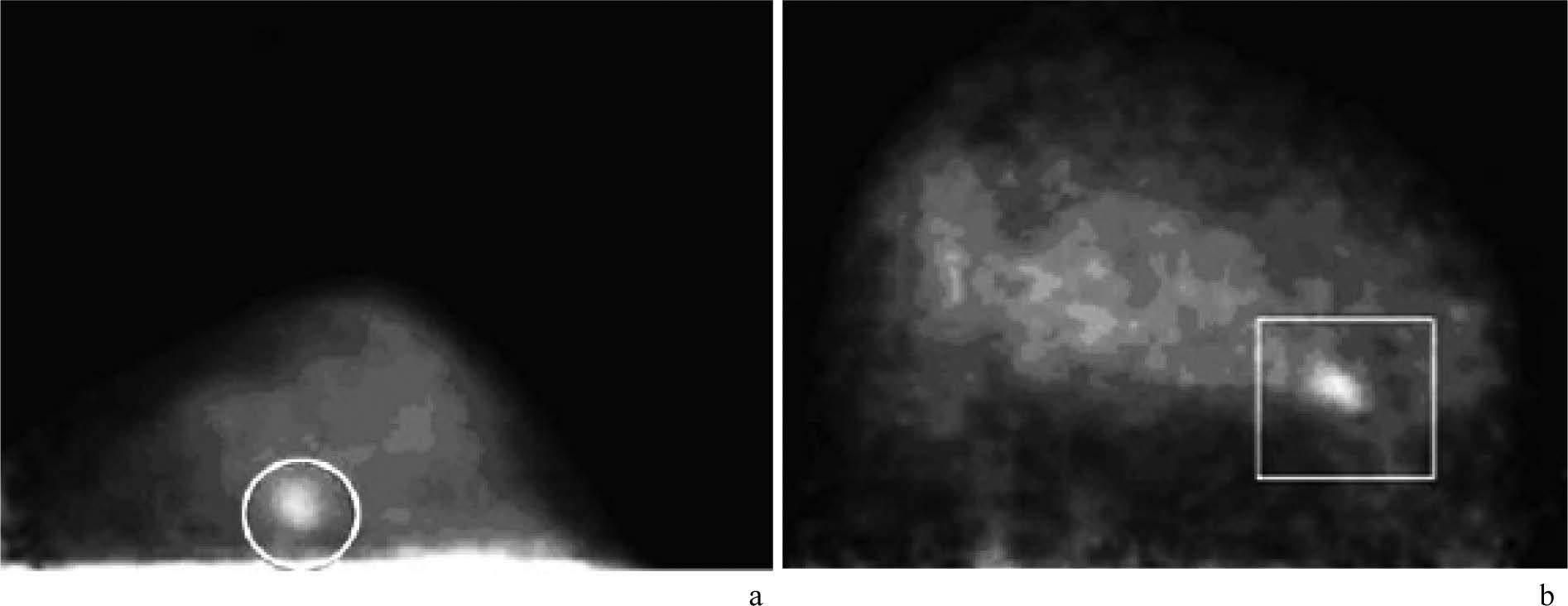

Figure 15: Demonstration of small invasive breast carcinomas with FDG PEM. Images from dedicated breast PEM units show 9-mm (circle in a) and 1.3-cm (rectangle in b) invasive carcinomas. (RadioGraphics 2007; 27:S215-S229)
Table 1: Summary of the Results of Published FDG PEM Studies

BSGI is the technique of using radiotracers (99m Tcsestamibi) to detect abnormal cellular metabolism, mainly of advantage in breast cancer detection. The highly advanced cancer enable optimized molecular breast imaging with a high-resolution, small field of view (FOV) detector for image acquisition used breast specific gamma camera. The devicehas a high sensitivity (96.4%).20 The sensitiv- ity of BSGI is comparable to MR (88%) imaging.21
The capability to help detect subcentimeter (defined as <1cm) cancer has been a criticism of gamma imaging of the breast. The sensitivity for subcentimeter lesion (88.9%) is higher than previously reported with scinti- mammography (35%-65%) and MR imaging (79.1%).21 In Rachel et al.20 study, five invasive cancers and three DCIS lesion measuring less than 5 mm were detected. The improved sensitivity with BSGI as compared with scintimammography results, at least in part, from the improved spatial resolution of the breast-specific gamma camera, as well as the decreased lesion-to-detector distance. The ability to detect small subcentimeter breast cancers should aid in the early detection of breast cancer, including occult foci. Additional studies with larger study populations are needed to further define the sensitivity of BSGI for both in situ and invasive subcen- timeter lesions.
Rachel et al.20 study demonstrates that BSGI has a sensitivity of 93.8% for the detection of DCIS and 97% for the detection of invasive cancers. This sensitivity is comparable to that reported in MR imaging for invasive cancers (90.9%) and better than that reported for DCIS (73%).21 An advantage of BSGI is the greater comfort of the patient, with the patient sitting as opposed to being placed in and MR imager. Additionally, BSGI results in four to eight images as compared with several hundred images in a breast MR examination, leading to a con- comitant decrease in interpretation time. The detection of occult cancers is an important goal of developing adjunct imaging modalities to mammography. In Rachel et al. study 7.2% of women with cancer were found to have additional foci of cancer that would not have been detected with more conventional imaging modalities. Given the high sensitivity of BSGI, it can be considered as a pre-surgical examination in patients with biopsy- proven cancer, to look for additional foci as well as contralateral breast cancer.
In conclusion, BSGI is a promising adjunct imaging modality with high sensitivity and moderate specificity to help detect breast cancers, including subcentimeter invasive and in situ cancers. Additional multiinstitutional studies are needed with larger study sample sizes to further evaluate BSGI.
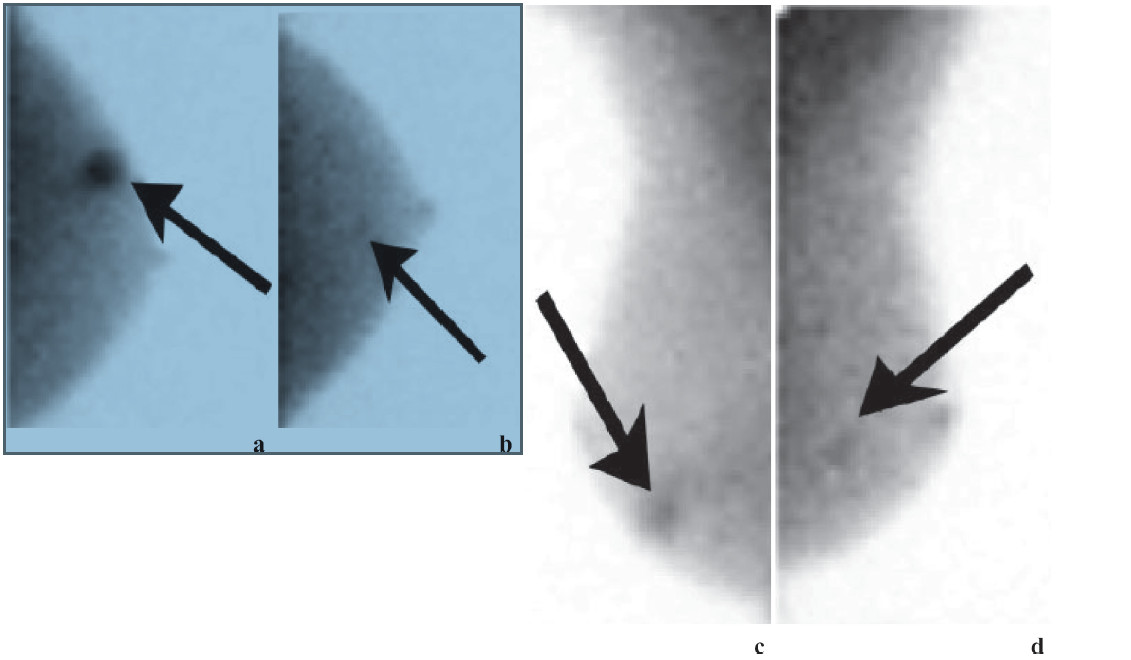

Figure 16: BSGI of 73-year-old woman shows vague mammographic architectural distortion in lower outer right breast. (a) CC and (b) MLO views show focal radiotracer uptake (arrow). Pathologic findings showed multifocal DCIS with no focus larger than 4 mm. Left breast (c) CC and (d) MLO views demonstrate small focal area of increased radiotracer uptake (arrows). Patient had normal mammogram and initial US findings. Second-look US identified vague hypoechoic area. Surgical excisional biopsy results showed single 4-mm focus of low- grade DCIS, which was occult and unidentified prior to BSGI.
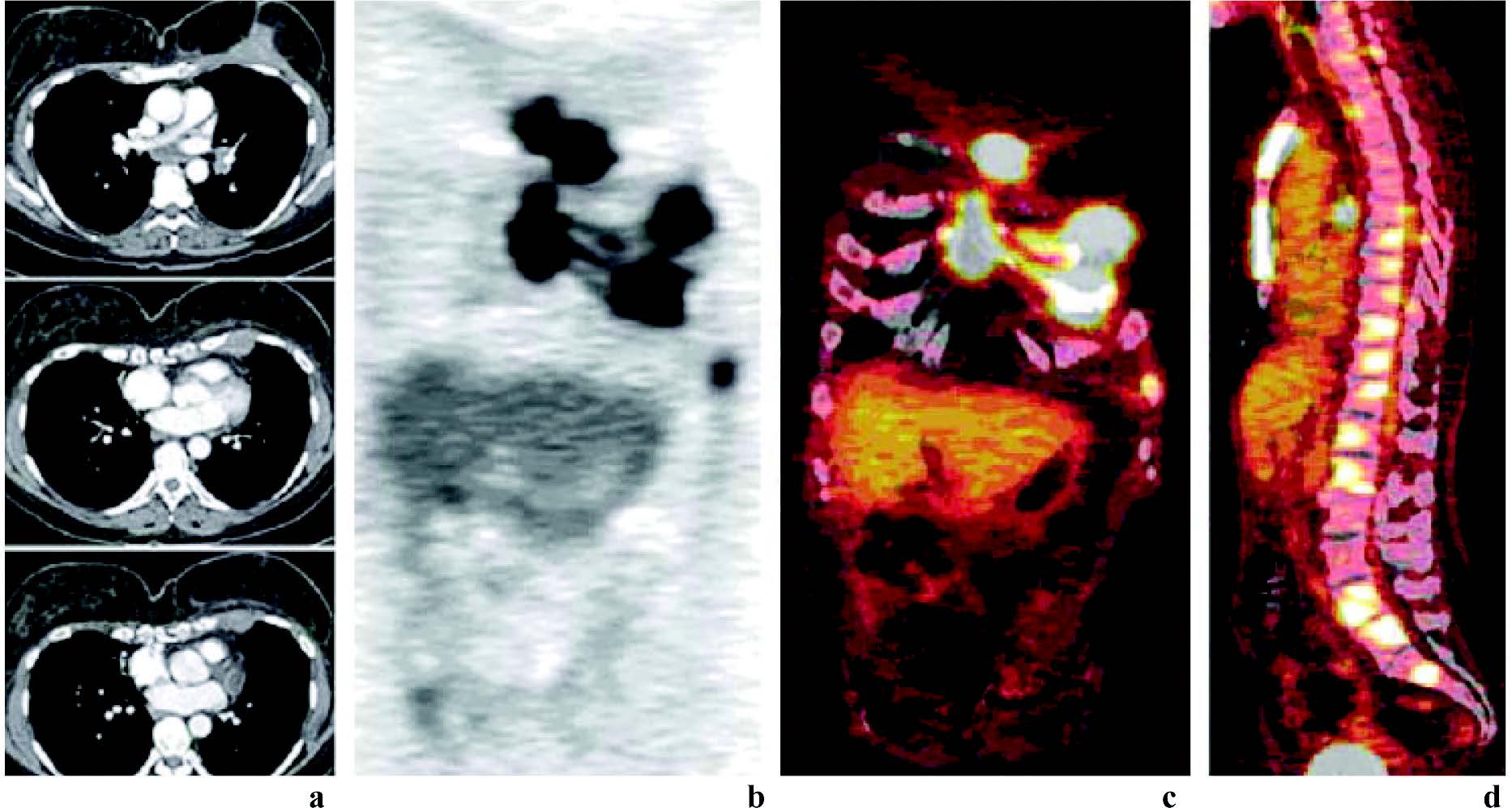

Figure 17: Results of PET/CT in a patient suspected of having recurrent breast carcinoma. Axial contrast-enhanced CT (a), coronal PET (b), coronal fusion (c), and sagittal fusion (d) images show extensive local recurrence involving the breast, sternum, anterior chest wall, and pleura as well as hilar metastases and diffuse bone metastases. (RadioGraphic Octerber 2007 volume 27)
Positron emission tomography (PET) and combined PET/computed tomography (CT) are increasingly used for oncologic imaging. Fluorodeoxyglucose (FDG) PET demonstrates abnormal metabolic features associated with malignancy, that often precede morphologic find- ings demonstrated with anatomical imaging. Combined PET/CT systems are increasingly available and currently account for almost all of the new whole body PET instal- lations. In these systems, the CT and PET images are fused and provide combined anatomic and physiologic imaging. Typically, the CT portion is used to provided attenuation correction as well as anatomic correlation for the PET imaging component. This modality allows more precise anatomic localization of PET abnormalities and in general has been shown to improve diagnostic accu- racy compared with FDG PET alone.
Further studies comparing FDG PET to sentinel lymph node biopsy support sentinel lymph node biopsy for early-stage disease and confirm the relatively low sensitivity of FDG PET for axillary nodal metastases in early-stage breast cancer. FDG PET and FDG PET/CT can improve staging and alter therapeutic options in patients suspected to have breast cancer recurrence and distant metastatic disease, primarily by demonstrating local or distant nodule involvement occult at other imaging studies.
It appears that FDG PET is complementary to bone scintigraphy, which remains the standard imaging proce- dure for surveying the skeleton for metastatic involve- ment. FDG PET can provide additional information in staging or restaging cases when results of conventional imaging are equivocal or conflicting. It also can be used in setting to evaluate the response of metastatic breast cancer to systemic therapy, since conventional imaging is often challenging in this setting.
In conclusion, FDG PET and PET/CT have been shown to be most helpful in staging recurrent or meta- static breast cancer. Emerging data support the use of FDG PET/CT in advanced axillary disease and evalua- tion of regional nodal spread in locally advanced breast carcinoma. FDG PET is complementary to conventional staging procedures and should not be a replacement for either bone scintigraphy or diagnostic CT. FDG PET and PET/CT have been shown to be particularly useful in the restaging of breast cancer, in evaluation of response to therapy, and as problemsolving method when results of conventional imaging are equivocal. In these situations, FDG PET often demonstrates locoregional or unsuspected distant disease that affects management. PET has demonstrated a particular capability for evaluation of chemotherapy response in both patients with locally advanced breast carcinoma and those with metastatic disease.16
Over the last century, the uses of mammogram for detecting breast cancer have come a long way. With the advances in imaging technology and understanding of cancer biology, earlier breast cancer detection has given direct benefits in terms of clinical outcomes. As mam- mography remains the standard of care for breast cancer screening, new innovations in mammography promise to advance understanding of the disease and the tumor’s response to treatments, but also may integrate into existing platforms for potential ease of access and lower costs.
The innovations of mammography starting from analog mammography using on target and material in x-ray tube and then the direct and indirect conversion technique integrated to develop the full-field ditigital mammography (FFDM). This innovation shows high resolution image and becomes popular in the efficiency for PAC system which provides teleconsultation. In the mean-time, computed radiography (CR) develops a dedicated CR reader for general mammographic purposes. The CR mammography provides image comparable with FFDM. The cost of investment is lower than FFDM. The early detection for anatomic and physi- ologic change of early breast carcinoma are feasible. The DBT demonstrates mass accurately. It is more useful in dense breast. In the meantime US, develops a high resolution transducer which able to measure elastoplasty in mechanical and automatic shear waves. The physio-pathologic study based on neoangiogenesis and cellular metabolism as follow MRI with Gd study, CE-DSM and CE-DBT. The cellular metabolic changes are nuclear medicine for instance breast specific gamma image (BSGI). The research for early detection of early breast carcinoma is going on and nowaday, the researchers aim to detect the abnormality at molecular level.