The purpose of a scientific article is to communicate the results of a research project1. Just imagine, you have recently invested a great deal of effort in designing a study, getting approval from the EthicsCommittee, registering participants, collecting good data, and spent a lot of time analyzing it. Now you want to share the results and conclusion with your colleagues in the form of a publication. It is important that your article is well-written, concise, clear, simple, and easy to understand2,3. Your readers can then follow your train of thought and be convinced by your conclusion.
Although the structure of articles from different journals may differ somewhat, most of them have a similar basic structure. What is called an abstract in one journal may be called a summary in another. What is called methods in one publication may be called materials and methods in another. Regardless of the name used, the reason for this basic structure is that people have found it to be helpful for a logical presentation of research.
In this article, the authors outline this structure and then explain theindividual components following the order of a typical scientific paper.
In order to make the article easy to read, it is necessary to have a goodunderstanding of the importance of the paragraphs. Knowledge of the grammatical structure of phrases is also required. A scientific article is effective when it is coherent in both substance and form. This allows the reader to follow the author’s logic.
The following is required for a good writing style:
1. Use the active rather than the passive voice.
2. Write short sentences. Excessively elaborate and long sentences are harder to understand.
3. Start a new paragraph when the subject changes.
4. Write in a logical sequence in accordance with the original research questions.
The internal consistency of the article depends on the organization and sequence of the paper based on the research question and the possible resulting answers. For example, the introduction should review the literature and guide the reader toward a research question. The methods section is organized based upon the order of the hypotheses and objectives. The results section follows the order of the methods section. The discussion is structured according to the results. Consequently, the reader knows what can be found in each section of the text (Table 1).
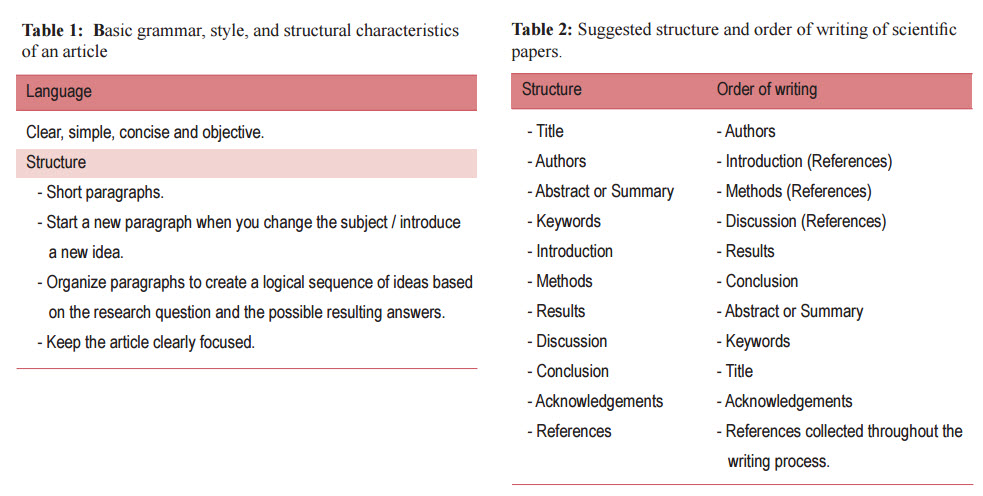
The best way to understand the structure of a paper is to study the articles in a scientific journal. Most likely, you will find that each article consists of sections arranged in the following order with some slight variations (Table 2):
1. Title
2. Authors
3. Abstract or Summary
4. Keywords
5. Introduction
6. Methods
7. Results
8. Discussion
9. Conclusion
10. Acknowledgements
11. References
Although this is how scientific articles appear in print, they were certainly written in a different order. The actual order of writing is more of a personal preference than an exact science. Some advocate starting with the results section while others insist on the introduction or the methods section. Usually the sequence of the writing happens in accordance with the steps of the ongoing research (Table 2). The following order of writing is uggested:
1. Authors
2. Introduction (+References therein)
3. Methods (+References therein)
4. Discussion (+References therein)
5. Results
6. Conclusion
7. Abstract or Summary
8. Keywords
9. Title
10. Acknowledgements
11. References collected throughout the writing process
Before you start writing, select a target journal for your manuscript. Different journals have different presentation styles and instructions. By adhering to the guidelines of the target journal from the beginning, one can save a lot of time and energy in revising the format of a manuscript later.Professional medical writers support can be associated with more complete reporting of results and higher quality of written English4.
A scientific article is written in a different order from the actual reading of the article. The description of the sequence
of the writing is presented here in accordance with the steps of the ongoing research: Authors, Introduction, Methods, Discussion, Results, Conclusion, Abstract or Summary, Key words, Title, Acknowledgements, and References.
1. Authors
The researchers should define before the study begins who will be the manuscript authors or who will be acknowledged even if adjustments may be needed later. At the same time,every author’s contribution and responsibility should be defined5.
Since authorship often becomes a contentious issue, guidelines on this subject have been developed by various groups. In general, authors must have made substantial scientific and intellectual contributions (such as contributions to hypothesis formulation, study design, statistical analysis, interpretation, and discussion) to the study. All authors have to approve the final version of the manuscript and agree to be accountable forall aspects of the work. For further information, the International Committee of Medical Journal Editors’ guideline (ICMJE, http://www.icmje.org/recommendations/browse/roles-andresponsibilities/defining-the-role-of-authors-and-contributors.html) may be consulted.
Conflict of interest can subconsciously influence theauthors throughout the study. Declarations of financial support to the authors promote transparency and help the readers indeciding about the validity of the study.
2. Introduction – What is the question?
The introduction justifies the study. It describes the objectives and contains brief statements on its purpose. It identifies current knowledge gaps and anticipates how the study results may close it. The methodology used and the diagnostic hypothesis should also be introduced in this section. These items will also be covered later in the methods and discussion sections.
The length of the introduction will depend on the length and the complexity of the project. Generally it is around half a page and no more than one page. Do not feel obliged to cite all of the papers ever written on the subject in the introduction. Not only does this bore the readers and cause them to lose interest in reading your article, it also suggests that the author does not know how to prioritize.
Introductions are oftentimes written in the present tense because they report what is known at the time the article is written, what is not known, and what the authors intended to study. It is not advisable to write in the first person, e.g. “we did” or “we were able to confirm that.”
Consider writing the introduction in three paragraphs:
1. First paragraph - Background. Review what is generally known about the topic in the literature. Include the most relevant literature. Questions to be answered: What? What is the topic of the study? What are the characteristics and causes of the chosen topic?
2. Second paragraph - Justification for the study. Emphasize the unknown aspects of the subject and describe the problems to be studied. Controversies in the literature should be introduced and the clinical question formulated. Questions to be answered: Why? What is its purpose? What are the objectives behind developing the study? What is the focus to be developed?
3. Third paragraph - Objectives and Hypothesis.Explain the logic of your hypothesis and develop clear research questions. In case more than one research question is presented, primary and secondary objectives should be presented separately. Briefly introduce the methodology and the strategy. Questions to be answered: How? What is the method to be used in the work? What methodology or strategy will be used?
3. Methods – Explain the Study Design
Although it may seem that the methods section is verysimple to write, it is nevertheless crucial for the success of a manuscript.
A well-elaborated methods section may convince the reviewers of the validity of the study design, the reliability and competence of the research team, and thus the reproducibility of the results. If other researchers apply the same methods under the same conditions, the results should be similar.
The methods section should be written in the past tense because it describes the actions that have already been taken. As much as possible, it should follow a chronological sequence so that the reader will find it easier to understand. For well-established procedures, instead of writing everything out in detail, it is advisable to reference published work to save time and space. Bibliographical citations are generally not included in the methods section, unless scales or other measuring instruments that have been published previously are used.
There is no clear limit regarding the number of pages of the materials and methods section, but it is essential to make it as concise as possible. Many authors underestimate how difficult it is to do so properly. It is very important to get this section right because it allows the readers to evaluate the results and, at the same time, makes it easier to understand the study described in the paper.
The methods section must be well-organized and related to the general and specific objectives of the project6,7. Use the same division or subsections to describe the results and discussions. Consider dividing the methods section into the following subsections to make it more transparent and easier to read:
a) Research design. Tell the readers how the study was performed. Include details such as:
- Study design, eg, prospective or retrospective.
- Justification of the choice of methods and techniques.
- Duration and follow up period.
- Place (eg, single or multicenter) and environment (eg, trauma center or rural hospital) of the study.
- Ethics committee approval number, informed
consent, trial registration numbers.
b) Study population. Describe details such as how patients were recruited, the size of the sample, and the inclusion and exclusion criteria. If the sample is subdivided into groups, these must be defined, as well as the system adopted for the randomization, if applicable.
c) Intervention and control group. Introduce the protocol, such as the techniques used and the measuring methods, in chronological order following the criteria below:
- For new protocols: provide step-by-step descriptions in detail.
- For published protocols: briefly describe the procedures and refer to the original publications.
- For modified existing protocols: explain the modifications in detail and refer to the original publications.
It is recommended to use the product’s generic name in a report on a clinical study and to avoid use of the commercial name. Whenever commercially available products (or products under clinical investigation) are used for a study, product brand names, their manufacturers and manufacturing locations need to be mentioned at least once in the manuscript.
d) Measurement. State the primary and secondary endpoints, how each outcome was measured, and the time points for measurements. The data collection methods and measuring instruments used in the study have to be described. References for the measuring methods, including their validation, should be given. For measuring instruments, include information such as the model, the calibration system, and the manufacturer. Clinical charts, opinion surveys or other measurement instruments could be included as annexes at the end of the paper.
e) Statistical analysis. Specify the statistical method used for sample calculation and the statistical tests selected for the study. If appropriate, indicate the computer software used to analyze the results8.
4. Discussion
Part 1: How are the results interpreted?
The structure of the discussion can be written even before all of the study results are available.The main objective of the discussion is to place the research findings in the context investigated, explain their meaning, and emphasize their importance as compared to the findings and the opinion of authors who have already researched the field or topic.
The discussion section is the most difficult and complex part of a scientific article because it showcases the researcher’s understanding of the topic. The background must be written in the present tense and the description of the research findings in the simple past.
The discussions should be constructed hierarchically: start with the most important (primary) result and contrast it with the available evidence. Then address the secondary objectives and their results in paragraphs that follow. Whenever possible, the content should be put into the same divisions or subheadings used in the methods and results sections.
Consider the following structure which you can start writing even before the results section is available:
a) The first paragraph reports the most important findings of the current study and their impact on the topic being investigated. The present results, particularly when seen in the light of previously reported results, should be interpreted. Differences to the general information or prior knowledge (accompanied by the bibliographies) should be emphasized and the results compared. This helps the authors to establish their position regarding the questions formulated in the introduction and to defend their thesis. It also allows the authors to transform their hypothesis into a conclusion later. The scientific relevance, the negative and positive points of the articles referenced should also be pointed out atthis point. Depending on the number of previous studies presented, multiple paragraphs may be necessary.
b) The paragraphs following the first paragraph interpret and analyze the results in the light of general information, always accompanied by the respective bibliographies.It is important to emphasize the scientific relevance of the article and the negative and positive points of the study. Following the same structure, present and discuss the secondary outcomes when applicable.
c) The second-last paragraph highlights the study’s weaknesses and strengths. It is obvious that the strengths of the study should be addressed---this helps to convince the readers of the validity of the conclusions drawn. The favorable points of the study should be strongly emphasized, describing and strengthening the new aspects discovered and the gaps in the literature that were answered by the study. Declaring the study’s limitations and weaknesses, however, is equally important. If you do not critique your study yourself, it is likely that the reviewers will do it. Explain the reasons why such limitations exist. This monstrates
one’s firm grasp of the methodology and the subjectmatter, and avoids potential reviewer objections.
d) The last paragraph may comment on future implications and the next research study proposed by the research group based on the present investigation.
Part 2: How are the results interpreted?
After all the results have been obtained, the findings arecompared with the results from other studies previously described in the literature. At this point, the discussion will transform the hypothesis into a conclusion, because based on the results, the author establishes his position regarding the question formulated at the beginning of the work, and defends his thesis. In this phase, there is flexibility for the author to express his opinions.
Authors should avoid presenting additional information (results or details of methods) that was not touched upon in the work, independent of their relevance. Discussing aspects not presented in the present study, irrespective of their relevance, is also inappropriate. Not only do such debates lack substance and lead to no conclusion, they generally draw criticism from reviewers and editors.
5. Results - Reporting the Findings of the Study
This section includes a clear, objective description of the findings and must be related to the structure of the methods section. Presenting results in the same order as the methods section and organizing the text with titles and subheadings make the results easier to follow and easier to read.
The purpose of the results section is to report whathappened during the study, present the findings, explain any deviations from what was planned in the study, and supply a visual or graphic summary of the study flow. Results are always presented in the past tense.
A study flowchart showing patient enrollment, allocation, follow-up and analysis helps the readers to get an overview on how the study was conducted. Deviations from the study plan should be explained in the results section.
Relevant results that prove or reject the study hypothesis should be communicated to the readers in clear, objective language. This means using numbers instead of adjectives (eg,very, rarely, often, generally) whenever possible. Self-explanatory tables, graphs, figures, and images can help simplify data presentation. Information from tables or figures should not simply be repeated in the text. Instead, emphasize the main findings that will later be aligned with the hypothesis and the objectives in the discussion section. There is less misinterpretation if the author provides absolute numbers and lets the reader interpret for him/herself whether the phenomenon is rare, very rare, or infrequent.
Authors should avoid interpreting the findings or drawing conclusions in the results section. The former belongs to the discussion section, and the latter to the conclusion section.
6. Conclusion
The conclusion summarizes the study outcomes in brief statements. These statements should include how the findings advance the current understanding of the topic, their impact on future research directions and clinical practices. A reliable and convincing conclusion can only come from a well-written,well-designed and well-conducted work. Avoid re-analyzing the results at this point. Neither self-aggrandizement nor apologies for the imperfections of the work is appropriate in this section.
7. Abstract or Summary
An abstract is a stand-alone part of a manuscript and should contain the summary of all relevant results and conclusions. It should only contain information presented in the full article,therefore we strongly advise that it be written last. Most journals limit the length of an abstract to 250-500 words, and may prescribe a structure such as the following:
- Introduction: the background of the clinical question
and the main purpose of the work must be clearly written.
- Methods: the study design and stages used to achieve
the objective(s).
- Results: the main findings and their analyses.
- Conclusion: how the results answer the primaryresearch question.
8. Keywords
Keywords, in addition to the title, provide another way to alert readers to your work. Selecting keywords that are Medical Subject Headings (MeSH) terms can help raise thevisibility of your paper.
9. Title
While the title is the last part of the article to be written, it is not the least important. A title is the first thing that editors, and later, the potential readers, encounter. Therefore it must attract the readers’ attention and at the same time be accurate, informative, and complete.
A title is basically an extremely simplified and condensed description of a study. Elements belonging to a title include the main topic, study design (eg, randomized trial, cohort study, prospective versus retrospective study), number of patients, main outcome, and follow-up time. A successful title should attract the target audience, whether they are doing a database search or browsing the table of contents of a journal, so that they want to go on to read the full article. The words selected for the title are essential for the initial evaluation of the article, and for the article to be found by the researcher who is interested in the subject.
Appealing titles are usually precise, specific, and quite short. Avoid having words and phrases in the title which do not contain useful information. These include constructions such as: “about”, “presentation of a new case of”, “considerations about“, “contributions to the knowledge on”, “study of”, “study about”, “influence of”, “interest of”, “investigations about”, “our personal experience with”, “new contributions to”, “observations on”, and “about the nature of”.
10. Acknowledgements Many people may have helped with the research and prepared the manuscript for publication, but do not qualify as authors based on the ICMJE guidelines. Their contributions should be acknowledged in this section. Examples of such contributions are: people who supplied special equipment or provided substantial technical help, who provided major help writing the manuscript, and who critically read and commented on the manuscript, but did not participate in the planning, implementation and drafting stages of the article.
Grants and funding may be acknowledged in this section. Some journals may require that these be declared in either the
methods section or a separate conflict of interest section.
11. References
References provide the basis for everything in the manuscript that is being conveyed to the reader, and reference management should be in place even before the research begins. Reference management software can help to catalog, organize, and format the references according to the publication rules of different scientific journals.
References are best compiled at the same time as one writes the manuscript (see the “Basic Structure of a Scientific Paper”
section). Constantly inserting references as the paragraphs are written saves time. It also helps to avoid situations in which
some references are left out or mixed up. Authors should always know precisely from where the data were extracted when using references.
In medicine, many international book publishers and periodicals, especially North American, follow the standards proposed by the ICMJE. We advise using a referencing program because this makes adapting to different journal styles much easier.
The recommendations needed for writing an article adequately can be summarized in four points: be clear, accurate, consistent, and logical. The best way to learn to write is in the writing itself. Aside from that, paying attention while reading other articles and asking for feedback from experienced and asking for feedback from experienced writers also helps.
The editorial obligations according to each Company must also be respected, like the presentation of the bibliographical references (for Springer, the year of publication of the reference must obligatorily appear between brackets behind the name
of the last author and not at the end of the reference), the presence of the sentence on the conflict of interests, and the sentence on the ethical rules.
Conflict of interest: Asdrubal Falavigna- nothing to disclose. Diarmuid De Faoite- nothing to disclose. Michael Blauth- nothing to disclose. Stephen L. Kates- grants from Research outside the submitted work.
Note: Research not involving Human Participants and/or Animals. Informed consent not necessary. Disclosure of potential conflicts of interest.
AO Program for Educations and Excellence in Research (PEER) group for the review support.