Arrhythmia is a crisis that patients need urgent treatment for since it affects the circulatory system and may cause death if the patients are not treated promptly. Amiodarone is an antiarrhythmia drug which is frequently used to treat patients with arrhythmia in the intensive care unit (ICU). Although intravenous amiodarone has been an important treatment for these patients, it often directly causes crystallization and irritation to the vascular walls which can be risk for vascular complications. Phlebitis is a common complication of intravenous amiodarone administration which is associated with pain, erythema, swelling, fever, and may increase the length of hospital stay and total treatment cost.1,2
There are several factors associated with the irritation to blood vessels such as high concentration, peripheral line, speed of administration, duration of amiodarone administration or the duration of catheter insertion, stability of drug, amiodarone pH, and host factors.3,4 Even though, the studies reported several contributing factors related to phlebitis and have recommendations for its administration, previous studies showed the incidence of amiodarone induced phlebitis vary, and range from 8-55%2 In Bangkok Hospital Rayong, a study of amiodarone induced phlebitis in the ICU during September 2015-January 2016 found a high incidence of 83.3%. Therefore, the purpose of this study was to determine the incidence and related factors of amiodarone induced phlebitis in patients receiving intravenous amiodarone in order to improve the amiodarone administration through a peripheral line provided for patients in the future.
The retrospective study was conducted at ICU Bangkok Hospital Rayong. The study was approved by the Institutional Review Board and Ethics Committee at Bangkok Hospital Rayong. Medical records of patients who received amiodarone between January 2011- December 2014 were examined among all patients who had amiodarone administered. The patients who were administered during resuscitation, and those who died or moved to other hospitals were excluded. Patient information in this research was treated with confidentiality. Phlebitis is a condition caused by inflammation of the veins, which causes pain, edema, erythema, and thrombus formation cording of the vein from intravenous infusion.5 The levels of phlebitis can be divided into 5 grades.6
• Grade 0: No Symptom
• Grade 1: Erythema at access site with or without pain
• Grade 2: Pain at access site with erythema and/or edema
• Grade 3: Pain at access site with erythema and/or edema, Streak formation, Palpable venous cord
• Grade 4: Pain at access site with erythema and/or edema, Streak formation, Palpable venous cord greater than one inch in length and Purulent drainage
Descriptive statistics were used to analyze concentration, type of solution, duration, peripheral intravenous size of catheter, age and underlying disease. Chi - square test was used to analyze the factors affecting the phlebitis.
From a total of 127 patients, 16 patients had phlebitis,an incidence of 12.6%. The duration of amiodarone administration was a significant factor associated with the onset of phlebitis (p = 0.001). Phlebitis occurred in 8 of 105 patients who were administered amiodarone ≤ 24 hours (7.6%) while 7 of 20 patients (35%) administered amiodarone > 24-48 hours and 1 of 2 patients (50.0 %) administered amiodarone> 48 hours presented with phlebitis.
No association was found between phlebitis and age,underlying disease, type of dilute solution, concentration, and catheter size. It was found that phlebitis occurred in 5 of 29 patients who had diabetes (17.2%), 9 of 63 who had hypertension (14.3%) and 2 of 54 cases who had other diseases (3.7%). According to the catheter size, none of the patients who were administrated amiodarone with a catheter size 24-Gauge presented with phlebitis while 11 of 60 those with catheter size 22-G (18.3%) and 4 of 29 those with catheter size larger than 22-G (13.8%) had phlebitis. Regarding the duration of amiodarone administration, 8 of 105 patients studied were administered amiodarone ≤ 24 hours (7.6%), while 7 of 20 patients administered amiodarone > 24-48 hours and 1 of 2 patients administered amiodarone > 48 hours (50.0%) presented with phlebitis (Table 1-2, Figure 1).
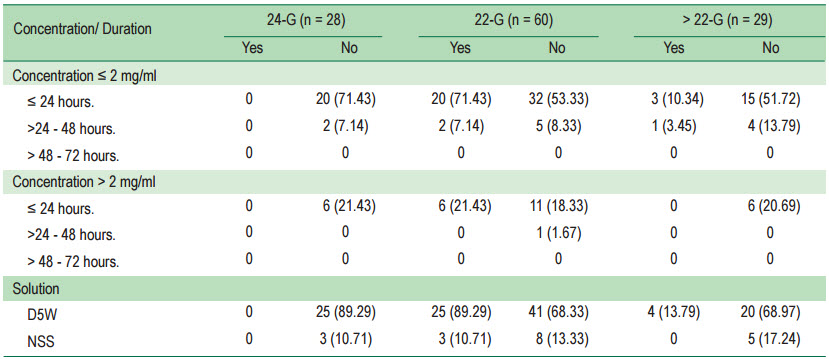
When considering the study of the incidence of phlebitis using size IV catheter in concentration, duration, and solution dilution no phlebitis occurred in catheter size no.24 (Table 2)
Table 1: The relationship factors affecting the incidence of phlebitis (n = 127)
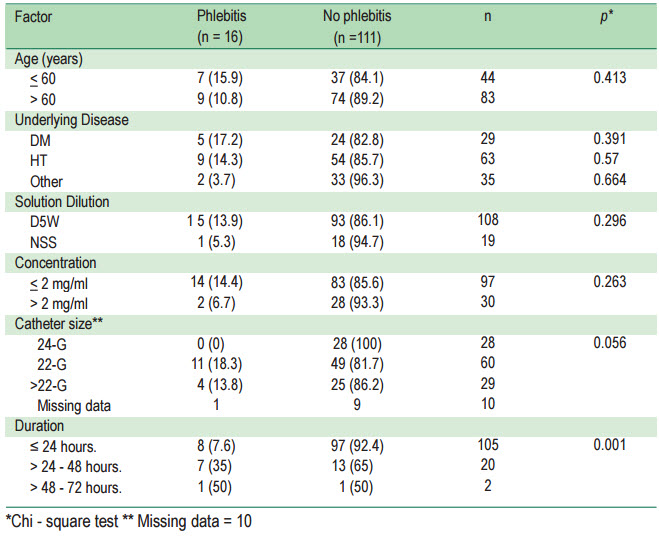
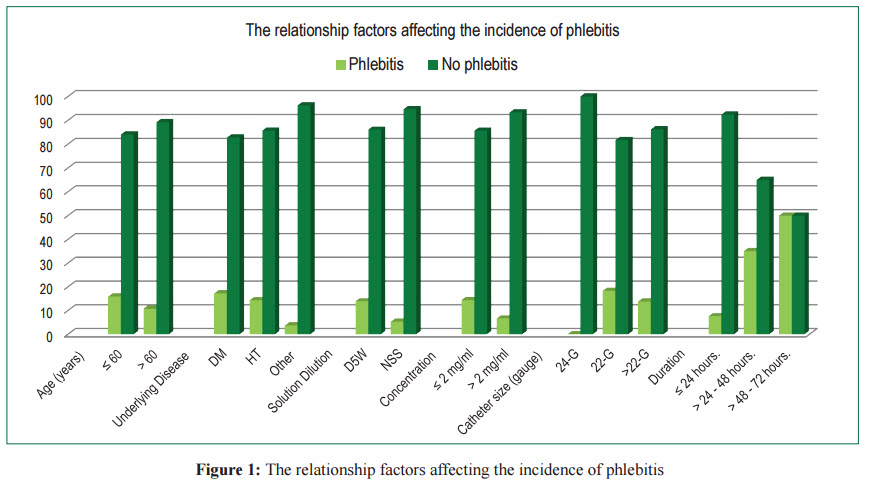
Table 2: Incidence of phlebitis using size IV catheter in concentration, duration, and solution dilution
According to the results, the incidence of phlebitis was 12.6% which differs from previous studies 7-55%.2,5 This may have been because the studies have differing research methods and amiodarone administration protocols. The important result of this study showed the duration of amiodarone administration was a significant factor associated with phlebitis. The duration of amiodarone administration above 24 hours increased the incidence of phlebitis. It is similar to the studies that showed a long duration of amiodarone administration was more likely to cause phlebitis.3
Although, the study revealed that phlebitis was not associated with age, underlying disease, type of dilute solution, concentration, and catheter size, it showed some interesting findings. In terms of catheter size, the result showed no evidence of phlebitis occurrence using catheter size 24-G regardless of duration, concentration and dilution. It can be concluded that amiodarone will aggregate and activate the blood cell components such as platelets, RBC or WBC as a focus causing phlebitis when using the catheter size 22-G and larger than 22-G.7,8 However when it comes to the smaller catheter size, the amiodarone may not activate this process because of more rapid flow in the smaller size catheter.
The concentration ≤ 2 mg/ml caused a 14.4% incidence of phlebitis, and the concentration >2 mg/ml caused a 6.7% incidence of phlebitis. Nevertheless, the review of relevant research studies revealed that the administration through a peripheral line with a concentration of less than 2 mg/ml would reduce the incidence of phlebitis. The results of the study showed that the concentration ≤ 2 mg/ml caused more phlebitis than the concentration >2 mg/ml. This may be because the concentration which was more than 2 mg/ml was administered only once and it was not continuously administered. As a result, it caused less phlebitis.
However the incidence of phlebitis may be due to multifactors and the review of relevant research cannot cover all factors affecting the incidence of phlebitis because any retrospective study has its limitations. Nevertheless, the research studies revealed that the use of a smaller catheter size and the administration of amiodarone in under 24 hours decreased the incidence of phlebitis. Therefore we developed guidelines to regulate the administration of amiodarone in the intensive care unit of Bangkok Hospital Rayong. We are planning a prospective study to determine the incidence of Phlebitis in patients receiving amiodarone administration, taking into account other factors and the limitations on this study in order to continuously develop and improve the amiodarone administration guidelines
In the incidence of phlebitis occurrence by amiodarone intravenous administration at peripheral entry mainly radial vein and few via cubital vein, we found that phlebitis will not occur when using a catheter size 24-G regardless of the concentration, duration and dilution (mixed with DW or NSS). The explanation is that a more faster velocity in administration achieved in catheter size 24-G when compared to the larger size of catheter means that the amiodarone will not aggregate and combine with platelets, white blood cells and red blood cells to cause phlebitis. The duration of amiodarone administration above 24 hours results in an increase in the incidence of phlebitis.
The researchers would like to express their sincere gratitude to the Bangkok Health Research Center (BHRC) in providing useful advice and recommendations which contributed to the success of the research. In addition, the researchers would like to extend their sincere thanks to the executives of Bangkok Hospital Rayong who gave the opportunity and support to conduct this research and also to all those who provided assistance, support and encouragement in doing this research