Mammography and ultrasound are common anatomical imaging procedures used to detect breast cancer. While screening mammography, especially when combined with ultrasound, has demonstrated the ability to detect non-palpable breast cancer, these modalities still suffer from some significant limitations. According to the results of the American College of Radiology Imaging Network (ARCIN) 6666 clinical trial, the combination of mammography and ultrasound resulted in a positive predictive value of only 11.2% and a missed breast cancer in 8 of the 40 (sensitivity 80%) participants with malignant lesions.1 In recent years, molecular imaging technologies have been developed to address these limitations.
Breast-specific Gamma Imaging (BSGI), also referred to as molecular breast imaging (MBI), scintimammography or mammoscintigraphy, is a nuclear medicine breast imaging technique which has been significantly improved within recent years with the invention of breast optimized gamma camera designs. Prior to this development, such studies were generally conducted with standard, large fieldof-view (LFOV) gamma cameras. Nearly 100 peer-reviewed papers dating back more than 15 years have documented the experience of this imaging technique using Tc99m methoxyisobutylisonitrile, (Sestamibi) and several studies comparing standard and optimized camera designs have provided evidence that the breast-optimized designs improve the clinical accuracy of this procedure, especially in sensitivity for sub-centimeter lesions.2-5 Clinical evidence proving the increased lesion sensitivity of Breast-Specific Gamma Imaging (BSGI) over scintimammography is now available.6-8 In addition, there have been several publications indicating that the sensitivity and specificity for BSGI are both around 89 - 96% and 65 - 90% respectively.9, 10 Although alternate pharmaceuticals are available and others are under investigation, Sestamibi is currently the only US FDA (Food and Drug Administration) approved single-gamma emission isotope approved for breast imaging.11, 12
According to the Society of Nuclear Medicine Guideline for Breast Scintigraphy with Breast-Specific Gamma Cameras released in 2010, There are several potential indications for the use of this imaging technology.13
Patients with recently detected breast malignancy:
Patients at high risk for breast malignancy
Patients with indeterminate breast abnormalities and remaining diagnostic concerns
Patients with technically difficult breast imaging
Patients for whom Breast MRI would be indicated
a. implanted pacemakers or pumps
b. ferromagnetic surgical implants
c. risk of nephrogenic systemic fibrosis response to gadolinium.
d. body habitus exceeding the inside of the MRI bore
e. patients with breasts too large to be evaluated within the breast coil
f. patients with acute claustrophobia
g. other factors limiting compliance with a prescribed MRI study.
2. As an alternative for patients who meet MRI screening criteria: BRCA1, BRCA2 mutations; parent, sibling, or child BRCA+; Lifetime risk of 20-25% established; chest radiation between ages 10 and 30
Monitor neoadjuvant tumor response in patients undergoing preoperative chemotherapy
99mTc-hexakis-2-methoxyisobutylisonitrile, also known as Sestamibi or MIBI, was cleared by the US FDA in 1991 for cardiac perfusion studies. In 1996, breast imaging was added as an indication to the drug package insert following a clinical trial conducted with standard gamma cameras equipped with high-resolution collimators. According to the Dosage and Administration section of the drug package insert, breast imaging is to be conducted using a dose of 740 - 1110 MBq (20-30 mCi). This dose was determined largely by the low photon sensitivity of the imaging systems when equipped with highresolution collimators. Since that time, several breast optimized gamma camera systems have been developed with three times higher photon sensitivity.
99mTc-Sestamibi is a 140 KeV gamma ray emitting isotope in a lipophilic cation molecule. It is injected intravenously and is retained in cells by electronegative cellular and mitochondrial membrane potentials.14-16 Studies show that its accumulation is roughly proportional to blood flow, desmoplastic activity and cellular proliferation and therefore it accumulates preferentially in breast cancers compared with surrounding tissues.17-19 It is a lipophilic substrate for the P-glycoprotein (Pgp), a cellular efflux pump for various compounds.20-22 Therefore, Sestamibi exhibits rapid tumor wash-in (within about 2 minutes) followed by a slow tumor washout (over the course of several hours).23, 24 Based on these factors, imaging can begin within minutes after injection and can continue for up to about 90 minutes post injection, providing ample time for all required views to be conducted before the washout cycle negatively impacts lesion-to-background tracer concentration ratio.
According to the MIBI drug insert package and to the Society of Nuclear Medicine Breast Scintigraphy Guidelines released in 2004, a high-resolution collimator should be used for imaging with the standard gamma camera systems. This recommendation is due to the relatively large distances between the detector and the breast when the patient is in the prone, breast pendent position (Figure 1). The newer, breast optimized imaging systems allow the breast to be compressed directly against the detector system (Figure 2). This close imaging geometry maximizes spatial resolution and allows high-sensitivity collimators to be used.
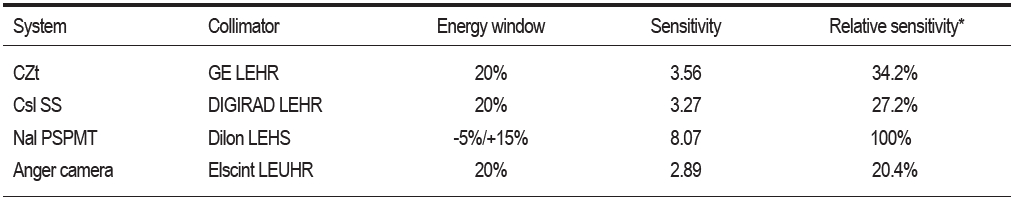
The Mayo Clinic conducted an analysis of detector performance for a number of commercially available breast-optimized detector systems. These results are shown in Table 1.25
Based on these results, the dedicated breast imaging systems provide a photon sensitivity up to 2.79 times greater than the standard gamma camera.
According to more recent publications, the Mayo clinic has continued to improve the design of the CZT detector system and according to data released in 2008,26 the photon sensitivity of this system is now approximately equal to that of the Dilon 6800. Thus both detector technologies should able to conduct breast imaging using approximately 35% of the usual dose or roughly 259-370 MBq (7-10 mCi).
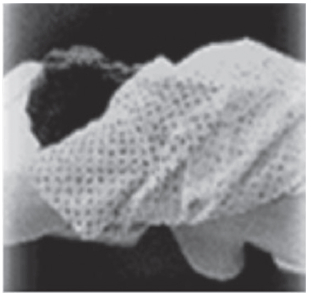
Figure 1: A patient positioned for breast imaging on a standard gamma camera system.
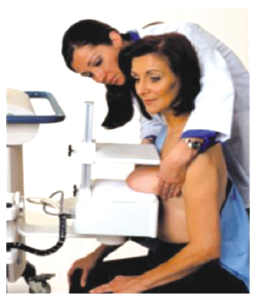
Figure 2: A patient positioned for imaging on a breast-optimized imaging system.
Table 1: System sensitivity (counts . min-1 kBq-1) comparison using the appropiate collimator and energy window for each system
In 2010, Böhm-Vélez et al initiated a prospective, IRB approved, clinical trial to examine breast tissue uptake as a function of injected dose.27 In their study, patients scheduled for breast scintigraphy as deemed clinically necessary by a referring physician had imaging conducted using a Dilon 6800 Gamma Camera, following the new Society of Nuclear Medicine Practice Guidelines for Breast Scintigraphy With Breast-Specific Gamma Cameras,28 but with one modification, each patient had a 740 MBq (20 mCi) dose separated into 2 syringes containing either two 370 MBq (10 mCi) doses or a 185 MBq (5 mCi) dose and a 555 MBq (15 mCi) dose. 43 subjects were randomized into receiving a fractional dose of 185 (n=14), 370 (n=14) or 555 (n=15) MBq followed by bilateral cranio-caudal (CC) acquisitions, (Figure 2). Then the remaining fraction of the 740 MBq dose was delivered and a normal 4-view imaging procedure was conducted, including an additional pair of CC images.
A region-of-interest (ROI) encompassing the breast was drawn for each of the CC images obtained from the low-dose and normal dose acquisitions and the mean number of counts per pixel (MCP) was calculated. Following correction of the MCP values for radioisotope decay and biological washout, the MCP of the first (low dose) image was expressed as a percentage of the MCP of the 740 Mbq image for each breast. The left and right breast percentages were averaged and compared to the activity of the first injected dose expressed as a percentage of the total injected dose.
Due to the time delay between first and second injections, corrections for radioactive decay and tissue redistribution, based on the model proposed by Del Vecchio et al29 were applied to the activity of the first dose when calculating the total activity at the time of the second injection.
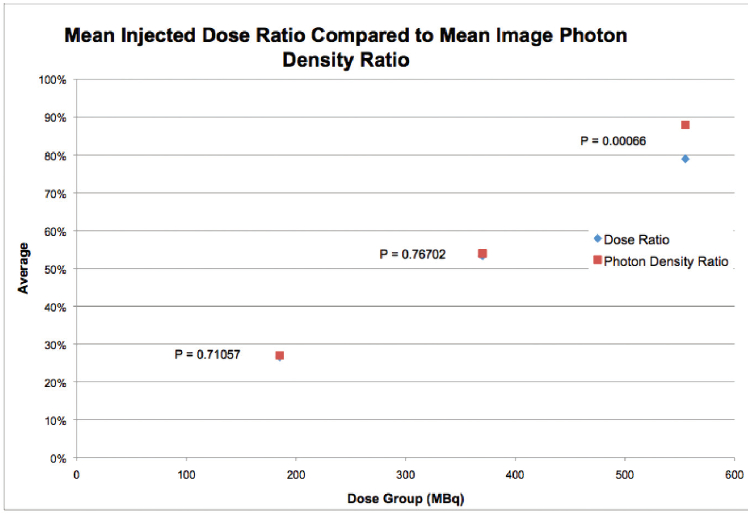
Figure 3: Graph plotted injected dose ratio compared to uptake ratio
As illustrated in Figure 3, for the first injected doses of 185 MBq, 370 MBq, and 555 MBq, the difference between the injected dose ratio and the measured uptake ratio was 0.4%, 0.6%, and 8.9%, respectively. Given the null hypothesis of equal uptake, the two-tailed t-test p values for these differences were 0.71, 0.76, and 0.0007, respectively.
These results suggest the uptake of Sestamibi in breast tissue may not be proportional to the injected dose, with decreased proportionality for higher injected doses. From this analysis of breast tissue uptake and image characteristics, it seems that the 555 MBq (15 mCi) dose provides nearly equivalent imaging to that obtained at 740 MBq (20 mCi). In addition, the breast tissue uptake at the lower doses of 370 and 185 MBq (10 and 5 mCi) appears to be more linear relative to the injected dose implying there is no obvious physiologic limitation to using these lower doses. It should be noted however that here is a broad variation in breast tissue uptake of +/- 30% of mean uptake value.
From the available data, it appears that these new detector technologies may reduce the dose required to conduct breast imaging with Tc99m-MIBI or dramatically improve image quality compared to those from the standard gamma camera systems using the same 740 MBq dose. Reducing the dose from 740-1110 MBq (20-30 mCI) to 259 -370 MBq (7 – 10 mCi) can reduce patient radiation exposure by nearly a factor of 3;however, as with all radiologic procedures, lower doses increase image noise which will make the visualization of small, low contrast lesions more difficult. The radiation exposure from a 259 MBq (7mCi) injection of MIBI is approximately 2 millisieverts (mSv) and is approximately equivalent to the radiation dose patients receive from the combination of screening and diagnostic mammograms. 30, 31
It is important to note that while several manufacturers of breast gamma cameras are promoting the use of doses as low as 74 - 148 MBq (2 - 4 mCi) there is no peerreviewed published clinical data to validate the sensitivity of low dose BSGI/MBI. Of the articles published on BSGI/MBI imaging more than 100 have been conducted using a dose of 740 MBq or more. Table 2 provides a comparison of the radiation dose patients receive from BSGI/MBI and other medical imaging procedures.
Table 2: The radiation dose from BSGI/MBI compared to other diagnostic imaging techniques.
There are several references available to help understand the potential health risks of radiation exposure. The first, according to a statement from the Health Physics Society “There is substantial and convincing scientific evidence for health risks following high-dose exposures. However, below 5 -10 rem (per year), risks of health effects are either too small to be observed or are nonexistent.”32 Using a dose of 740 MBq, this equates to approximately 8 BSGI/MBI studies in a single year or 16 in a lifetime. The United States National Institutes of Health’s radiation dose risk model estimates the likelihood of a radiation induced cancer from the 740 MBq dose of MIBI to be 24 cancers per 1,000,000 examinations or 0.024%.33
Table 2: The radiation dose from BSGI/MBI compared to other diagnostic imaging techniques.

Determining the optimal imaging dose is an important part of establishing an institutional protocol and several factors must be considered. The primary consideration should be a risk vs. benefit analysis based on specific indications. For the typical diagnostic patient, the primary concern should be image quality while for the asymptomatic screening patient; the radiation dose should be minimized. Secondly, one must consider how to address the relatively broad variation of breast tissue uptake. As aforementioned in the dose reduction section, there is a 60% (+/- 30%) variation in breast tissue uptake for any given dose delivered. Therefore the clinician must either choose a dose high enough to ensure good image quality for all patients or be willing to increase imaging time for patients on the low end of the uptake range.
Breast-Specific Gamma Imaging (BSGI) is a diagnostic breast imaging procedure becoming more common in clinical practice. The goal of this work is to quantify the clinical performance of BSGI against that of ultrasound in the management of patients who have a negative or indeterminate mammogram but require additional imaging.
A multi-center patient registry was maintained for all patients sent to BSGI as part of their diagnostic work up. From the registry data, patients who had a BIRADS 0, 1, 2 or 3 mammogram followed by ultrasound and BSGI were selected for evaluation. The BIRADS rating schematic was used for sonography and a similar, 5-category system was used for the BSGI images. For each modality, the reports were classified as negative BIRADS 0-3, or positive BIRADS 4, 5. Needle biopsy was conducted as deemed clinically necessary and lesions were classified as benign, malignant and high-risk (non-malignant pathologies requiring excisional biopsy). Pathology or a minimum of 6 months of follow up imaging was used as the gold standard.
A total of 190 lesions were evaluated in 188 patients; 155 benign, 27 malignant, and 8 high-risk. BSGI was positive in 24 malignant and 8 high-risk lesions and ultrasound was positive in 17 malignant and 4 high-risk lesions. The overall sensitivity for BSGI and ultrasound was 92% and 60% respectively while the specificity was 80% and 72% respectively. BSGI also performed better than ultrasound in terms of negative and positive value (52% vs. 32%) and (98% vs. 89%) respectively.
A 48-year-old with a previous negative biopsy of the right breast noted a dimpling near the nipple of her right breast during breast self-examination. The patient was seen for a mammogram (Figure 4) to evaluate the area of concern. No significant change from mammogram 2 years prior. No mass or spiculated lesion noted at the sight of the subtle dimpling and stable calcifications are noted in the medial aspect. Ultrasound shows a small cyst and a hypoechoic area likely representing scarring from the previous procedure. BIRADS 3. Patient was asked to return for additional imaging in 6 months.
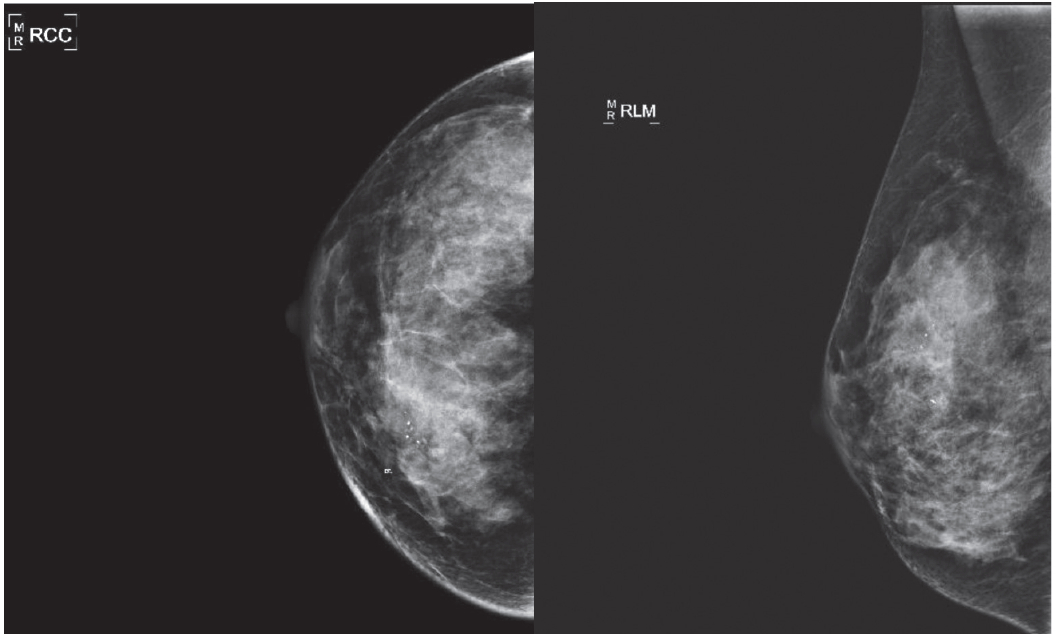
Figure 4: Mammograms revealed no mass or spiculated lesion noted at the sight of the subtle dimpling and stable calcifications.
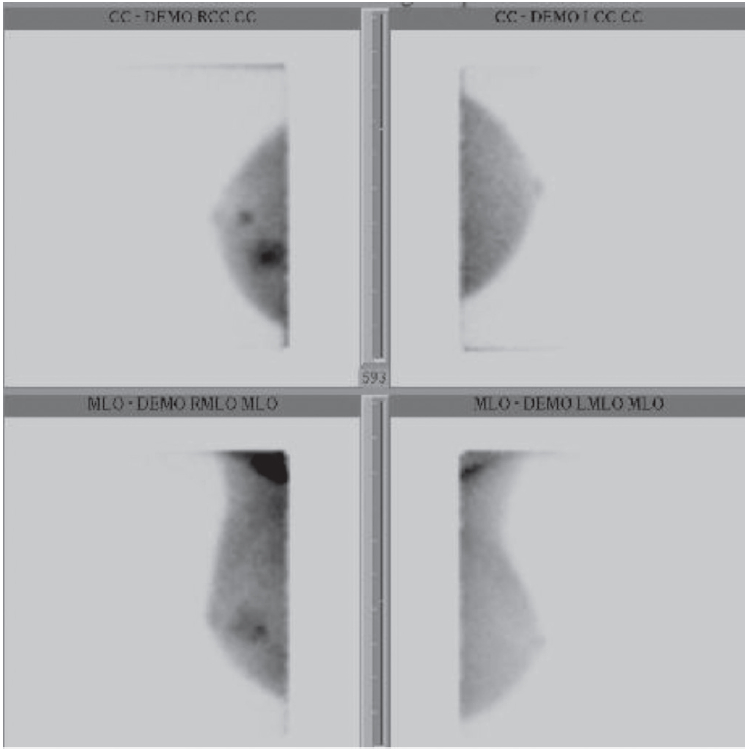
Figure 5: BSGI showed a large area of increase uptake in the upper inner quadrant and other small intense focus at retroareolar region of the right breast.
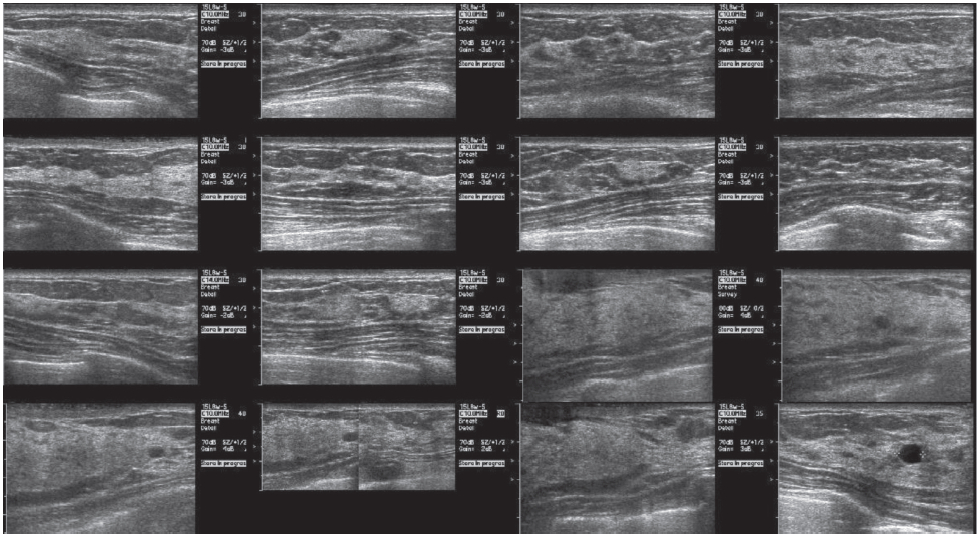
Figure 6: Second look ultrasound showed irregular hypoechoic mass at 6 o’clock position
8 weeks later the patient returned reporting an increase in dimpling. Clinical examination and sonographic evaluation of this area were negative leading to the suggestion of a BSGI for additional evaluation.
BSGI was utilized for this patient due to complex dense breast tissue, history of a benign right breast biopsy (upper inner quadrant), and a current complaint of dimpling in her right breast. The area of the palpable mass is stable on mammography and negative in clinical and ultrasonic examinations.
BSGI (Figure 5) of the left breast has normal, uniform distribution. The right breast has a large area of increased uptake in the upper-inner quadrant, measuring approximately 2 cm. A second, smaller and more intense focus is located retroareolar, measuring about 1 cm at the 6 o’clock position. In addition, there are areas of increased activity in the right axilla.
Second look ultrasound (Figure 6) was performed using the BSGI images as a reference and an irregular hypoechoic mass was noted in the 6 o’clock position consistent with the focal intensity noted in BSGI. Biopsy of this area was conducted resulting in a ductal carcinoma dignosis. In the area of the larger medial focal uptake on BSGI, ultrasound demonstrated fibroglandular tissue with complex cysts, but no mass or other distortion.
The large focal intensity noted in the upper inner quadrant of the right breast on BSGI was negative in ultrasound and corresponded to a cluster of stable microcalcifications visualized in the mammogram however, this uptake was still of concern to the surgeon who decided to place a localization wire marking the site of the microcalcifications under mammographic guidance prior to surgery in order to obtain tissue from this site at the time of the lumpectomy procedure for the retroareolar cancer. Pathology of this region revealed a 2 cm ductal carcinoma in-situ.
Patients who have a negative or indeterminate mammogram, but require additional imaging represent a difficult to manage group. This is especially true for patients with radiodense breast tissue which can easily obscure malignancies. Many times additional imaging is requested due to difficulty in interpretation of the mammogram, a lack of confidence in the technical sufficiency of the mammogram or difficulty in correlating radiographic results with clinical signs and symptoms. For this group of patients, BSGI can Figure 5: Second look ultrasound provide better sensitivity for the detection of breast malignancies than ultrasound while maintaining an equivalent specificity. Perhaps most importantly, the high negative predictive value of BSGI (98%) provides the clinician reasonable assurance that malignancy is not present in these difficult cases. Of the 190 patients imaged, 32 (17%) had a malignant or highrisk lesion requiring surgery but not detected by mammography. The theoretical risk for radiation-induced malignancy due to a BSGI study is 0.025% therefore the benefit (17%) substantially outweighs the risk (0.025%) by a factor of 680:1 for this patient population.