FDG-PET or positron Emission Tomography with Fluorode-oxyglucose (fluorine-18-2-fluoro-2-deoxy-D-glucose [FDG]) for oncologic imaging both detects and effectively monitors the therapy of many malignancies. FDG-PET improves the detection and staging of cancer, disease management, therapy selection, and the assessment of appropriate therapeutic responses. Besides the use of FDG-PET in oncology, it can also be used in neurology and cardiology. The clinical use of FDG-PET has flourished over the past 15 years. This article is not meant to be exhaustive, but rather to give an overview and to share some information and experience of FDG-PET and FDG production at Wattanosoth Hospital. It will touch briefly on all aspects of FDG-PET and its production, namely an overview of radioisotope production of F-18, FDG synthesis and quality control.
In the mid-1970s, Wolf et al1,2 successfully produced FDG, 18F-FDG or 2-[18F]fluoro-2-deoxy-D-glucose at the Brookhaven National Laboratory. It provides an impetus for the advancement of PET or Positron Emission Tomography which is now a powerful scientific and clinical tool for probing biochemical processes in human body. The medical community was excited by the possibilities of using FDG-PET in clinical applications, once the broad utility of this tracer had been demonstrated. Nowadays, FDG is the most widely used PET radiopharmaceutical. It is utilized largely in oncology, but also in cardiology and neurology and its use is steadily growing.3
FDG is a radiolabeled analog of glucose, in which the hydroxyl group on the 2-carbon of a glucose molecule is replaced by a fluoride atom. Just as glucose, it is taken up by glucose-using cells and phosphorylated by hexokinase as the first step toward glycolysis.4 However, FDG cannot undergo further metabolism and becomes effectively trapped intracellularly as FDG-6-Phosphate.
Whole body PET imaging with FDG measures glucose metabolism in all organ systems with a single examination. Since cancer is a systemic disease, FDG-PET allows the early detection and quantification of metastasis. Therefore, it has found applications in the diagnosis, staging, and restaging of several clinical conditions such as lung cancer, colorectal cancer, lymphoma, melanoma, head and neck cancer, brain tumor, breast cancer, and oesophageal cancer etc.5-7 Clinical applications of FDG-PET are also found in neurology, cardiology and inflammation/infection.
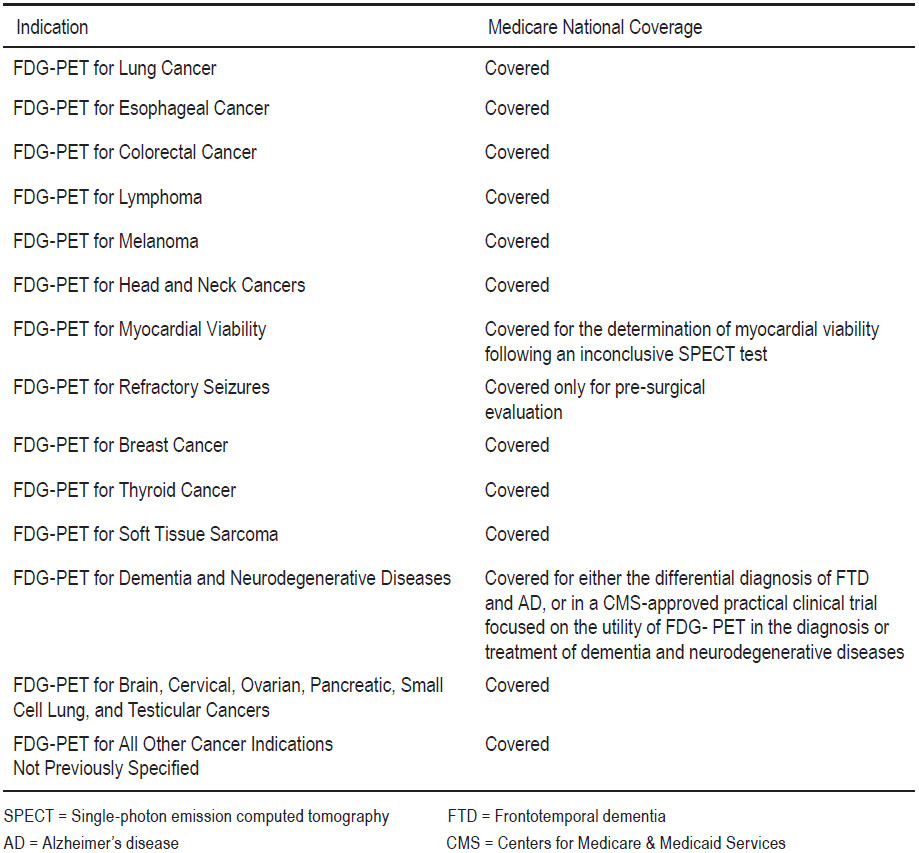
Table 1: Reimbursement of PET and PET/CT in the United States9
Although the PET imaging technique has existed for more than 30 years, it has been used clinically for only the last 10-15 years. There were many reasons for the dramatic growth of clinical usage of PET. Such factors are the approval of using FDG-PET by the US FDA, the availability of reimbursement in the late 1990s, the availability of medical cyclotron to produce the necessary short-lived positron emitters, the advancement in synthesis and quality control of FDG, and the invention of the combination of a dedicated PET scanner and multi-slice helical CT (PET/CT). PET/CT enables integrated functional and high-resolution morphologic imaging.
Since it was introduced to clinical medicine in 2001, PET/CT has represented one of the largest growth diagnostic modalities worldwide8. In the United States, the Centers for Medicare and Medicaid Services (CMS) allow reimbursement for most clinical indications in oncology, such as staging and restaging of lung cancer; esophageal, colorectal, and other gastrointestinal tract cancers; breast cancer; kidney and other genitourinary cancers; melanoma; head and neck cancers; and malignant lymphoma (Table 1)9. CMS state that more indications.
Buck et al.10 reported on the current knowledge of economic evaluations of PET and PET/CT in oncologic applications. “The clinical use of PET has been demonstrated to be cost effective for staging of non-small cell lung cancer, differential diagnosis of solitary pulmonary nodules, restaging of Hodgkin disease and non-Hodgkin lymphoma, and restaging of colorectal carcinoma”. The use of PET in clinical routines seems justified from a health economic point of view. Diagnostic effectiveness has been demonstrated for many other clinical indications such as monitoring response to therapy or radiation treatment planning. Buck et al go on to state: “PET and PET/CT are highly sensitive diagnostic tests to screen for metastatic tumor deposits in the entire body that may be missed by standard imaging modalities. The sensitivity for detection of lymph node metastases varies significantly among cancers and may be inferior compared with other techniques such as sentinel lymph node biopsy”.
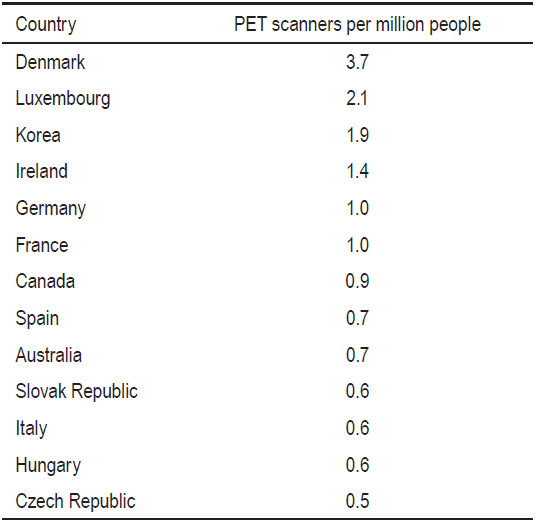
Public PET services are limited by high costs. Some countries such as Israel, France, Canada, Belgium and the United Kingdom (UK) rationalize public PET services by limiting the number of PET scanners based on population size.11 The number of scanners is controlled, which effectively controls the volume of PET scans, provided that each scanner operates at maximum capacity. The Royal College of Radiologists in the UK proposed that one PET scanner should be provided for populations of 1 to 1.5 million people.12 The World Health Organization (WHO) recommends two PET scanners for every million people. In 2009, approximately 2,000 PET/CT scanners were installed in the United States and approximately 350 were installed in Europe.10 In Germany, there were about 1.2 scanners per million people. While in the States, there were about 6.5 scanners per million people. In Hong Kong there were a total of 11 PET scanners in 2010, which represented 1.6 scanners per million. In Canada, the number of PET scanners per million people was 0.83 in the year 2009.13 A report on available PET scanners per million people as at 2007 (Table 2) is sourced from an international literature review on funding arrangements for diagnostic imaging services.14
In Thailand, there are total of 6 PET/CT scanners and 2 Cyclotron facilities in Bangkok since 2012. Two of PET/ CT scanners are in private hospitals. There is one cyclotron facility the in public sector and another one is in private sector. According to United Nations Thailand, the estimated population of Thailand in 2012 is 64 million of which approximately 9.3 million live in Bangkok and its vicinities. Therefore, there is about one PET scanner per 10.7 million people in Thailand (or 0.09 PET scannerper million of population) which is far below many other countries. The number of PET exams in Thailand is grow- ing slowly since the cost per PET exam is high and there are limited indications for reimbursement.
Table 2: Number of PET scanners per million people in various countries in 200714
The first PET/CT in Thailand was installed at Wattanosoth Hospital, Bangkok Hospital Medical Center in November 2005. The PET/CT scanner at Wattanosoth Hospital is a Gemini GXL. Up to November 2012, more than 6,000 PET exams have been performed at Wattanosoth. Most of these were FDG-PET for oncologic imaging; about 3% of the scans were for neurology imaging. However, the trend for using PET imaging in neurology is growing.
Patients are instructed to fast and not consume beverages, except for water, for at least 6 hours before the administration of FDG to decrease physiologic glucose levels and to reduce serum insulin levels to near basal levels. Intravenous fluids containing dextrose or parenteral feedings are also withheld for 6 hours. Patients are screened for a history of iodinated contrast material allergy, and renal disease, if intravenous contrast material is used. When the serum creatinine level is above 1.5 mg/ dL, intravenous contrast material will not be used. Blood glucose levels are checked before FDG administration. If the blood glucose level is greater than 200mg/dL, the patient will be rescheduled. The patient may be asked to consult an endocrine physician to control their blood glucose levels.
Prior to the administration of FDG, patients are assessed for any history of diabetes, their fasting state, current medication, any recent infections, type and site of malignancy, dates of diagnosis and treatment etc. Any history of claustrophobia and the patient’s ability to lie still for the duration of the acquisition (about 15-30 minutes) is investigated.
Adult patients will be intravenously administered about 6-14mCi of FDG according to their body weight. Children will receive about 6mCi of FDG intravenously. The radiation dose to the patient undergoing a PET/CT exam is the combination of the radiation dose from the PET radiopharmaceutical and the radiation dose from the CT portion of the study. The effective dose for an adult and a child of FDG is about 0.019 mSv/MBq (0.070 rems/ mCi) and 0.050 mSv/MBq (0.18 rems/mCi), respectively.15 The organ receiving the largest radiation dose is the bladder. The radiation dose to the bladder for an adult and a child are 0.16 mGy/MBq (0.59 rads/mCi), and 0.32 mGy/MBq (1.2 rads/mCi) respectively. The CT body scan may include various portions of the body and may useprotocols aimed at reducing the radiation dose to the patient or aimed at optimizing the CT scan for diagnostic proposes. Because of the wide diversity of applications, protocols, and CT systems, the effective dose from CT could range from approximately 5 to 80 mSv (0.5 - 8.0 rems).16
After administration, patients are asked to rest in a quiet room during the uptake phase to avoid muscular uptake for body imaging. For brain imaging, patients rest in a quiet and dimly lit room.
The image acquisition for FDG-PET is undertaken approximately an hour after administration. Patients are asked to void the bladder before the acquisition to limit the radiation dose to the renal collecting system and blad- der and for better image quality. Intense urinary bladder tracer activity degrades image quality and can confuse the interpretation of findings in the pelvis. Metabolic objects should be removed from the patient. For optimal imaging of the body, the arms should be elevated over the head if the patient can tolerate this position. Arms alongside may produce beam-hardening artifacts over the torso. That said, the arms should be positioned alongside for imaging of the head and neck. The PET emission image acquisition time is 5 minutes per bed position for body imaging. Typically, the PET imaging is done from skull to mid-thigh. The total acquisition time is about 20-25 minutes. The imaging time for the acquisition of a brain image is typically more prolonged.
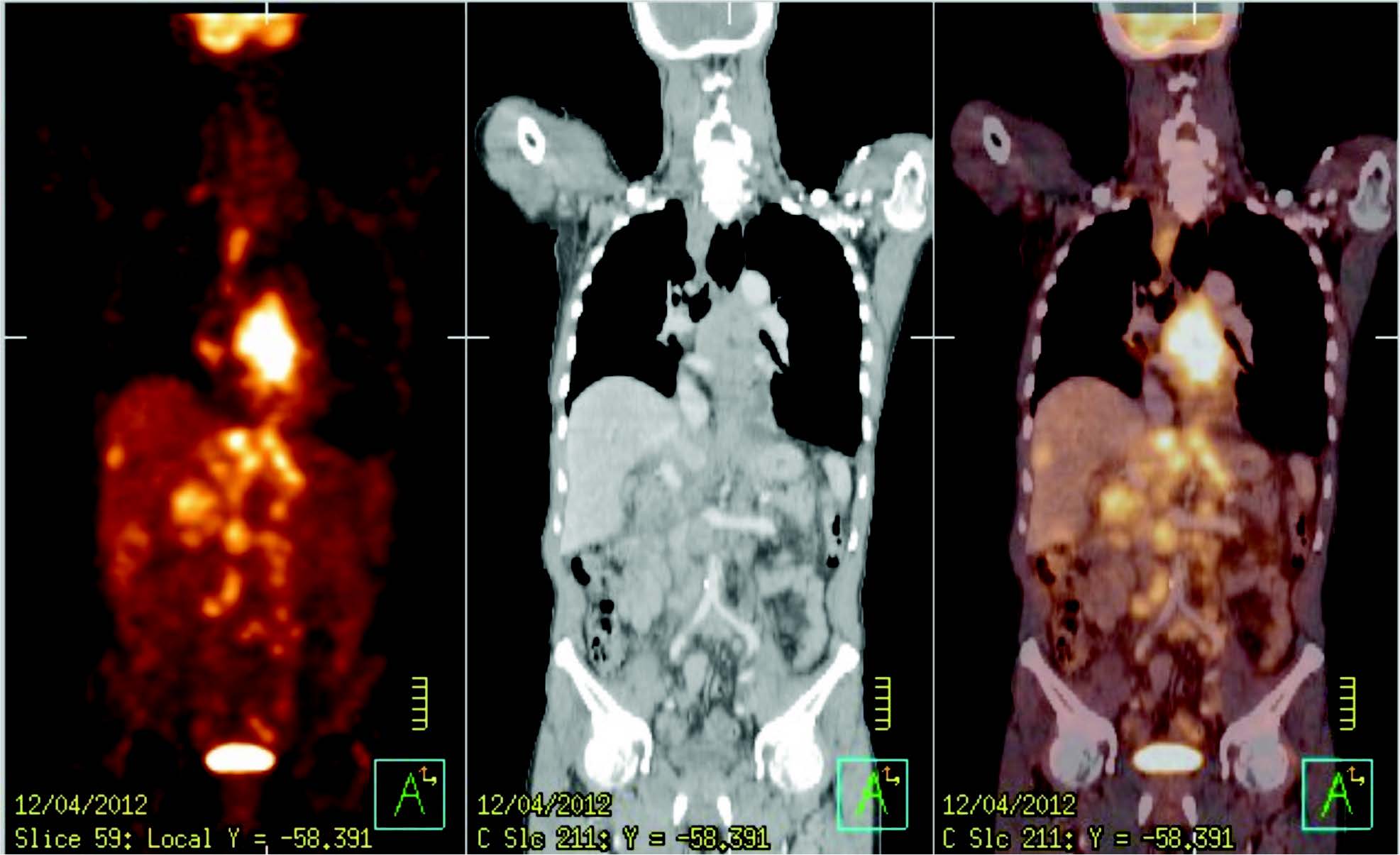
Figure 1: PET image, CT image and PET image fusion with CT image
PET images are reconstructed with and without CT-based attenuation correction. The oncologic FDG-PET/CT imaging is read by 2 different imaging experts (nuclear medicine physician and diagnostic radiologist). PET scans are interpreted by the nuclear medicine physicians with expertise in PET, and CT scans are interpreted by the diagnostic radiologists with expertise in CT. Strong opinions are summarized in a single report to avoid inconsistencies, confusion, and redundancy. Figure 1 shows a PET, CT and combined PET/CT images of a 63-year-old Thai male with esophagus cancer.
A summary of the physical characteristics of these PET radioisotopes and typical reactions of their production is listed below in Table 3. There are 4 most often used radioisotopes for PET imaging, namely fluorine-18 (F-18), carbon-11(C-11), nitrogen-13(N-13),and oxygen-15 (O-15).PET radioisotopes can be produced by either proton or deuteron reaction.
The most common PET radioisotope is typically produced by cyclotron. The first medical cyclotron was installed in 1941 at Washington University, St Louis, where radioactive isotopes of phosphorus, iron, arsenic and sulphur were produced. Nowadays, there are many commercial cyclotrons available (Table 4).17
Table 3: Physical characteristic of PET radioisotopes and reaction for production
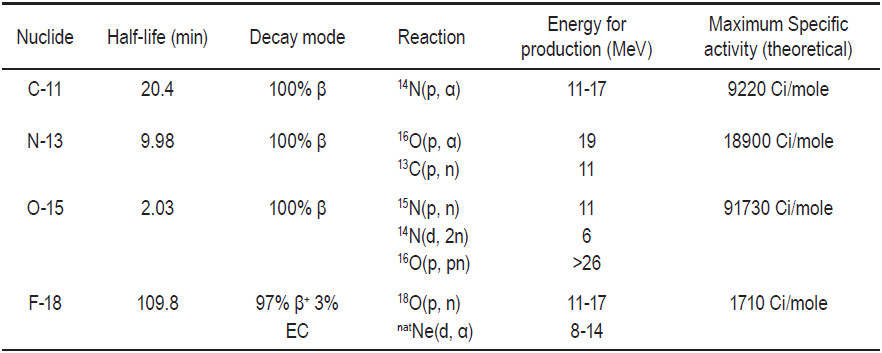
Table 4: Characteristics of some commercial cyclotron17
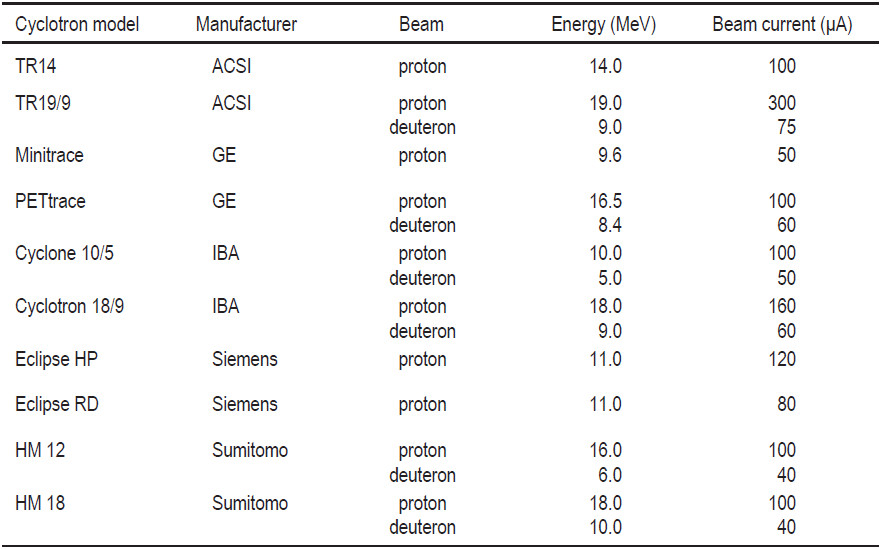
The range of radioisotopes produced and the quantities increase with particles energy. Table 5 shows popular radioisotopes that can be produced in each proton energies range.17 However, the increase in the number of radioisotopes that can be produced come at a cost both in equipment and in infrastructure. Moreover, as the energy increases, the number of side channel reactions increase and unwanted radioisotopes can be produced. This is especially true for beam energies greater than 30 MeV. Traditional radioisotopes used in nuclear medicine such as 201Tl, 67Ga, 123I and 111In have been produced via proton reactions for more than 25 years. Many of the most useful radioisotopes can be produced with proton energies of below 30-40 MeV. The selection of a cyclotron will depend on which radioisotopes are needed to prepare the radiopharmaceuticals used in the clinical and research programs, and on whether these radioisotopes will be distributed to other locations.
Establishing a cyclotron facility for producing radioisotopes and/or manufacturing radiopharmaceuticals is a complex process and requires careful planning in order to be successful. There are several essential considerations in development of such a facility, including design and technical aspects, feasibility studies and strategic planning, facility requirements and design, staffing, radiation protection, good manufacturing practices (GMP),and quality management.A facility for the production of radioisotopes and radiopharmaceu- ticals requires multi-disciplinary staff with a wide range of qualifications. Personnel required for the operation of a radioisotope facility includes cyclotron operator(s), production chemist(s), quality control (QC) chemist(s), a radiation safety officer, an electronic engineer, a mechanical engineer and a manager. In addition to the technological complexity, requiring highly skilled staff, it is also costly to build and operate. The main areas of such a facility are the cyclotron area, the hot laboratory areas, the dispensing areas and the QC laboratory. There are several relevant references in clinical literature describing the planning of new radiopharmaceutical production and/or PET facilities.18,19
Table 5: Popular radioisotopes that can be easily produced versus the proton energies required for their reaction17

Figure 2: TR-19 PET cyclotron
In June 2005, TR-19 PET, the first PET cyclotron in Thailand, was installed at Wattanosoth Hospital (Figure 2). It produces protons up to an energy of 19 MeV. Typical F-18 production uses proton beam energy of 18.7 MeV with a beam current approximately 80-95 μA. O-18 enriched water of 2.6 mL is loaded in the F-18 target chamber. The amount of F-18 that can be produced for a 30 minute irradiation is about 3 Ci. This amount is sufficient to produce FDG to supply all of the PET centers in Bangkok. The irradiation time for each production is calculated according to the amount of FDG needed. The TR-19 PET cyclotron at Wattanosoth Hospital can pro- duce F-18 up to 8 Ci of F-18 per irradiation period with the current set up. The TR-19 PET cyclotron at Wattanosoth Cancer Hospital is equipped with beamline which can be used for a solid target.
The radiopharmaceutical facility area at Wattanosoth Hospital includes a cyclotron vault, an equipment room, a control room, a changing room, an air lock room, a hot laboratory in a clean room environment, QC laboratory and dispensing areas. There are several potential hazards in a cyclotron facility. The normal hazards are high voltage, radiation, oxygen deficiency, high temperatures, and the movement of large pieces of equipment. All personnel receive training to understand these risks. There is an interlock protection system. Access to the accelerator or target is only allowed in a ‘machine off’ condition. The shielding around the cyclotron vault is there to reduce the neutron flux during machine operation. Any shielding that will reduce the neutron flux to an acceptable level will also reduce the gamma flux. The thickness of the shielding depends on the type of cyclotron, the energy, types of particles, and the targets to be used. Final testing of the shielding is done using protons on O-18 enriched water which produces a lot of neutrons.
To minimize the downtime of the cyclotron, regular preventive maintenance as well as proactive replacement of deteriorating parts is essential. Checklists occur weekly, monthly, quarterly and yearly for preventive maintenance according to the manufacturer’s recommendations. This ensures an effective maintenance regime. For production runs, a number of parameters are recorded for example environmental and facility parameter such as temperature and pressure of cooling water, room temperature and humidity, target pressure before and during irradiation, delivery times of activity to the hot cell, and a number of cyclotron running parameters. This checklist will help to detect gradual changes over time.
After cyclotron produces F-18 by proton beam irradiation of O-18 enriched water via 18O(p,n)18F reaction, the irradiated O-18 enriched water is then transferred from the target site to the synthesizer in the hot laboratory to proceed for FDG production. Currently, there are many commercially available FDG synthesis modules such as FASTlab, TRACERlab MX, BIOSCAN FDG synthesis Module, EBCO FDG synthesis module, Explora FDG 4, etc. Beside the synthesis modules, there are many other types of equipment needed in the hot laboratory such as hot cells, laminar flow hot cells or manipulation hot cells. Hot cells are exhausted and a shielded enclosure provides shielding for personnel against radiation from gamma emitters. The hot cells for the production of radiopharmaceuticals need to maintain negative pressure to prevent radioactive contamination. The hot cells should be leak tight according to accepted international standards. The wall of the hot cells should be smooth, impervious and unbroken and the corner curved.

Figure 3: Hot cells and a manipulation hot cell situated in a controlled clean room

Figure 4: HFASTlab
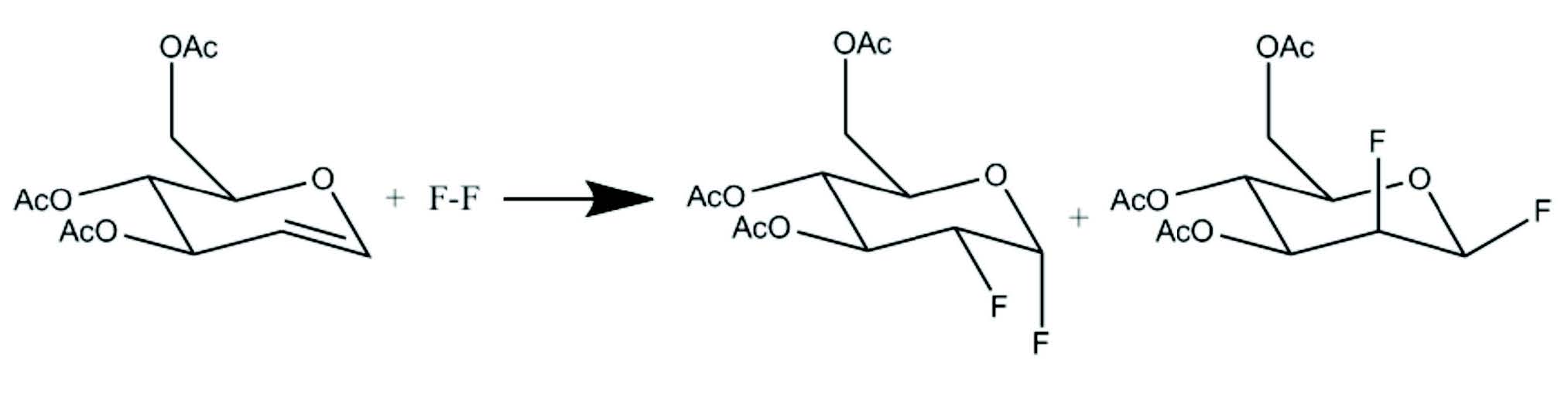
Figure 5: Electrophilic fl uorination
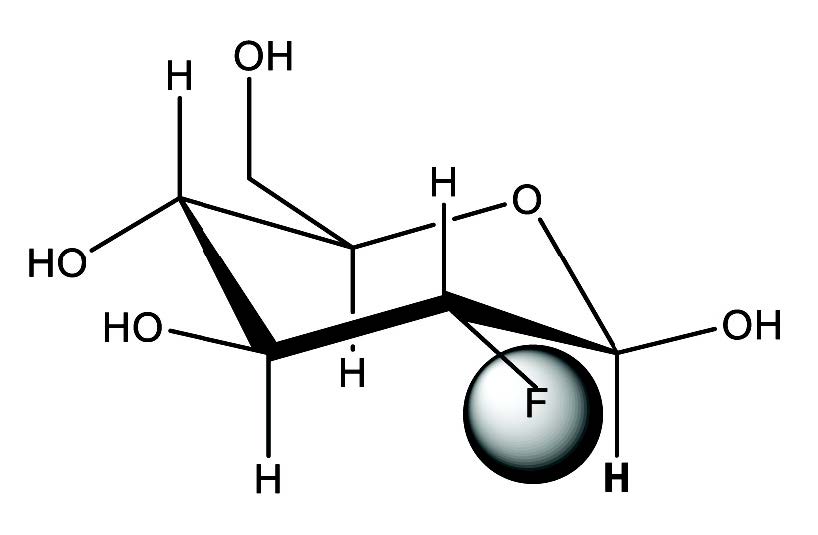
Figure 6: Structural formula of 2-[18F]fl uoro-2-deoxy-Dglucose or FDG
According to the recommendations for GMP, during their operation, the hot cells should be under negative pressure with a 20-fold air change per hour.18
The appropriate design and layout of a radiophar- maceutical production facility is an essential require- ment in achieving the desired product quality and safety. The laboratory design needs to take into account GMP for radiopharmaceutical production as well as radiation protection practices. The guidelines for the design and implementation of a radionuclide and radio- pharmaceutical production facility using cyclotron can be found elsewhere.18,20-24 However, there is currently no global harmonization of FDG quality specifications, or methodologies to achieve GMP compliance. For example, WHO and European Union (EU) regulations require compliance with guidelines applicable to conventional pharmaceutical manufacturing in addition to specific requirements for radiopharmaceuticals, necessitating these production activities be performed in environmentally controlled cleanrooms.22,23 On the other hand, in the U.S., production of FDG is governed by the GMP regulation designed specifically for PET radiopharmaceuticals.24 In Thailand, there is no specific regulation related to FDF production at present. The FDG production facility presented by IAEA (International Atomic Energy Agency) is based on GMP guidelines prescribed by WHO. The radiopharmaceutical production activities in Wattanosoth Hospital are performed in an environmentally-controlled clean room.
Currently, the hot laboratory in Wattanosoth Hospital is equipped with two single hot cells for FDG synthesis, one double hot cell for synthesis of C-11 radiopharmaceu- tical and a manipulation hot cell. Figure 3 shows the hot cells and manipulation hot cells in the hot laboratory. A hot cell with tele-manipulators and a double hot cell will be installed next year.
In 2005 and early 2006, there was only one FDG synthesis module by EBCO (currently ACSI), called Single FDG Synthesis Module. A year after its initial installation, another FDG synthesis module called Double Synthesis Module from ACSI was installed. The synthesis time for EBCO FDG synthesis modules (both Single and Double) is about 35 minutes. The uncorrected yields of FDG were approximately 40% and 50% at the end of synthesis for single synthesis module and double synthesis module respectively. Since then, the demand for FDG is growing rapidly throughout the world, and many companies keep improving the efficiency of FDG synthesis modules. In the beginning of 2012, FASTlab by GE was installed in the hot laboratory. The FDG synthesis time for FASTlab is about 25 minutes with approximately 70% yield of FDG. FASTlab is equipped with an integrated cassette pre-loaded with reagents that simply snaps into place. The system design offers consistently high reproducibility. Figure 4 shows a chemist installing a cassette into FASTlab set in hot cells.
The first synthesis of FDG was carried out in Brookhaven National Laboratory by Wolf et al25 in 1976 by electrophilic fluorination. Electrophilic fluorination refers to the addition of fluorine atoms across a double bond, producing a difluoro derivative of the present compound as shown in Figure 5. The structure of FDG is shown in Figure 6. The synthesis took 2 hours and the yield was 8%.25 Despite the low yield and long synthesis time, the Brookhaven team was able to collaborate with the Hospital of the University of Pennsylvania to map glucose metabolism in human brain. Several improve- ments to electrophilic fluorination were made thereafter. The major limitation of electrophilic fluorination was that only 50% of the radioactive fluorine atoms were incorporated into the precursors. The specific activity is low due to the presence of the non-radioactive fluorine gas since the 18F-F2 was produced from a Neon gas target with 0.1% to 1% of fluorine gas via a 20Ne(d,α)18F reaction. Moreover, the maintenance and operation of a Neon target is troublesome. The yield of 18F- with the 20Ne(d,α)18F reaction was much lower than with the 18O(p,n)18F reaction26.
Many attempts have been made to develop a nucleo- philic substitution for synthesis of FDG. But the major breakthrough was reported in 1986 by Hamacher et al. who used Kryptofix 222TM as a catalyst.27 The reaction time is shortened to 50 minutes and the yield of FDG is over 50%. Nucleophilic substitution is a chemical reaction involving the addition of a nucleophilic molecule (highly negatively charged molecule) into a molecule with a leaving group (electron drawing group attached to the parent molecule through an unstable chemical bond). In the synthesis of FDG, 18F ion is the nucleophile. The precursor for FDG is manosetriflate. In mannose triflate, the 1,3,4,6 carbon positions of a mannose molecule are protected with an acetyl group and triflate is the leaving group at the 2-carbon. In the presence of Kryptofix 222TM as catalyst and acetronitrile as solvent, 18F ion approaches the mannose triflate at the 2-carbon, while the triflate group leaves the protected mannose molecule to form FDG (Figure 7). Details on FDG synthesis can be found elsewhere.20,28 At present, com- mercially available computer controlled automatic synthesizers use a nucleophilic process for FDG synthesis. Purification of the final FDG can be performed with a series of anion exchange column, a C-18 reverse phase column and an alumina column.
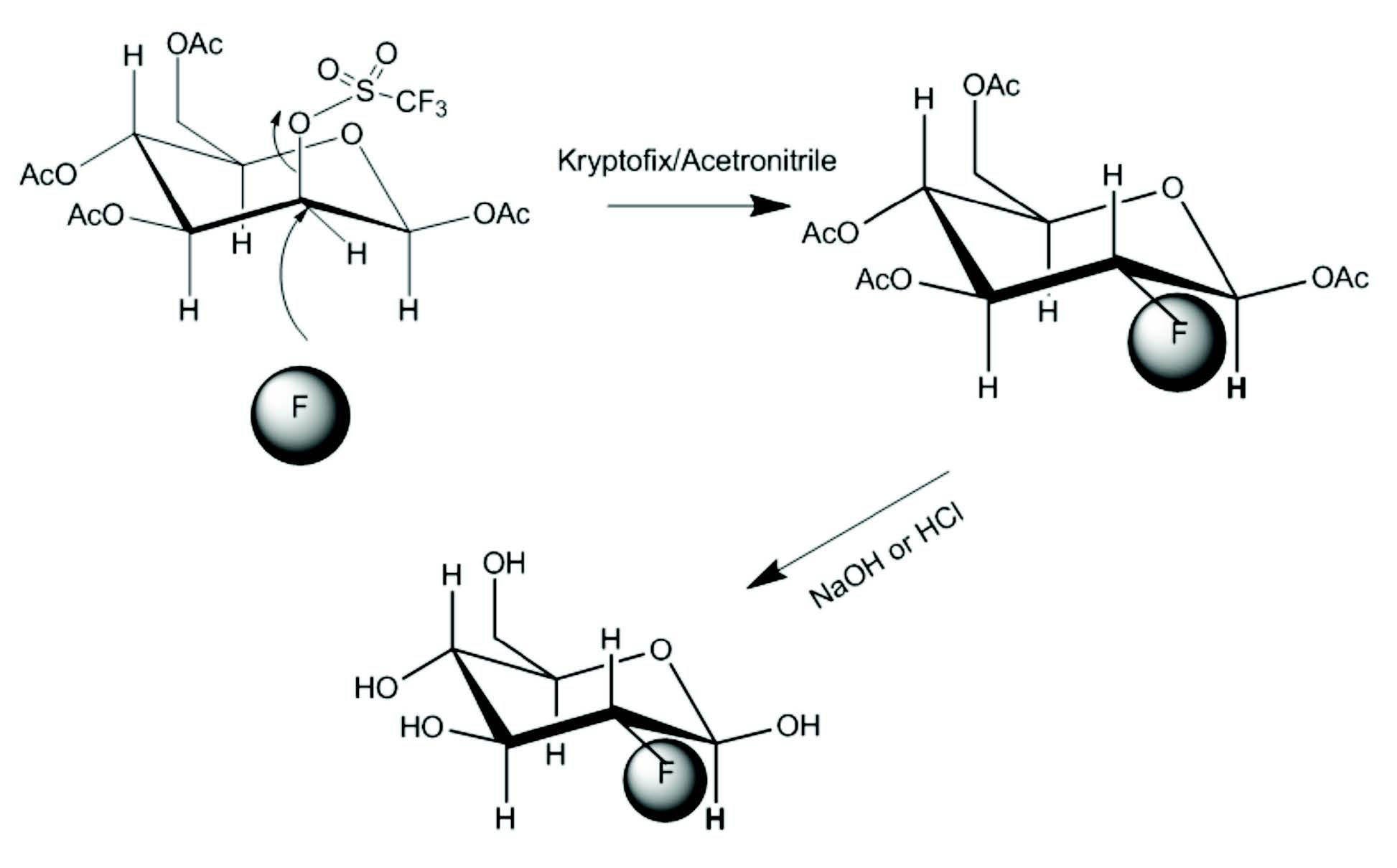
Figure 7: FDG synthesis by nucleophilic fluorination
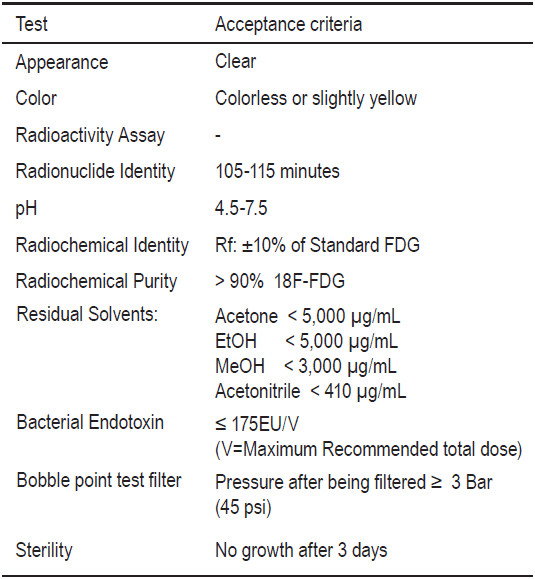
Table 6: Example of quality control criteria for FDG
Since it was first installed and up to November 2012, Wattanosoth Hospital has produced 1,312 batches of FDG production with total of 6,695 doses of FDG. FDG produced here was supplied not only to Wattanosoth Hospital but also many other hospitals in Bangkok namely, Bumrungrad Hospital, Chulalongkorn Hospital, Chulab- horn Hospital, Siriraj Hospital, and Ramathibodi Hospital.
FDG must conform to various quality attributes of purity, efficacy and safety prior to being considered suitable for patient use. There are some minor differences in FDG quality specifications described by three major pharmacopoeias, namely the International Pharmacopoeia (Ph.Int.), the European Pharmacopoeia (Ph. Eur.), and the United States Pharmacopoeia (USP).29-31 FDG speci- fication is not currently specified in Thai Pharmacopoeia.
Since the half-life of 18F is 110 minutes, it is necessary for the application of a QC test procedure to be completed in a relatively short time period. But, not all tests can be completed prior to the release of a product for patient use, namely sterility. In spite of this restriction, all tests except sterility can be performed within 30 minutes of post-production. Application of GMP, strong quality management and a validation of the entire system ensure a safe and high quality product. Table 6 shows the example of quality control test criteria for FDG used at Wattanosoth Hospital. Most of the tests are done before the release of FDG to patients.
Quality testing of FDG prior to release for patient use is a major component of manufacturing, but quality control (QC) alone does not constitute good manufacturing practice (GMP). Greater confidence in product quality can only be achieved through strict adherence to GMP guidelines during batch manufacturing. Radiopharma-ceuticals are pharmaceutical products and must therefore be manufactured according to the basic principles of GMP. GMP encompasses everything that has a bearing on the quality of the pharmaceutical product including personnel, premises equipment, starting material, processes, QC/QA (quality assurance), documentation, packaging and shipping. In the past, there was no specific GMP guideline for radiopharmaceutical manufacturing. In contrast to conventional pharmaceuticals, the presence of the radioactive component in a radiopharmaceutical adds complications to the manufacturing process and controlrequirements. Specific differences include radia- tion hazard to the operator, and a relatively short shelf-life of several radiopharmaceutical products due to the short half-life of the radioisotope incorporated within the radiopharmaceuticals. Moreover, radiopharmaceutical products are often used in a patient prior to full assessment of quality. Therefore, quality assurance in these cases is highly dependent on the adherence to GMP protocols and procedures. Recent guidelines for radiopharmaceutical products can be found elsewhere.32-34
FDG is the most used radiopharmaceutical in PET. It is aptly named the ‘Molecule of the Millennium’ due to its versatility and enormous important application in oncology, neurology and cardiology. PET continues to be the primary functional imaging technique for conduction translational research studies aimed at identifying the molecular basis of human disease. The future of PET will depend on the application of upcoming new radiophar- maceuticals and the regulatory framework for the usage and approval of new PET drug products. Establishing a PET center and radiopharmaceutical facility is a complex process. Investment and operation costs are high. Moreover, a number ofhighly qualified personnel is needed. Most PET centers and cyclotron facilities, especially where clinical research will be conducted, need collaboration among many specialties. Nuclear medicine physicians, nurses, technicians, physicists, organic/medicinal chemists, chemical engineers, mechanical engineers, analytical chemists, and radio pharmacists need to work in concert to meet the significant time constraints imposed by the short-lived radioisotopes routinely used in PET.
However, the number of PET scans for each scan- ner is still small. There are only 6 PET centers in Thai- land at present and the number of PET scanners per million people in Thailand is far fewer than many other countries. The limited indication for reimbursement is one of the important factors for the underutilization of PET scanners in Thailand. Besides this, the knowledge of medical personnel to use PET is also a factor. Governmental support to maximize the use of existing facilities is crucial for improving the healthcare of the Thai population.
Acknowledgements
The author thanks the PET center and Cyclotron facility team at Wattanosoth Cancer Center including doctors, nurses, physicists, technologists, chemists, engineers and pharmacists. During the past 7 years, they have put a lot of effort into continued improvements in quality and range of services. Also, the author is very grateful for the support from the executive of the Bangkok Hospital Medical Center and Wattanosoth Cancer Center.