A case of penetrating aortic ulcer (PAU) with an aorto-iliac occlusion from aortic dissection (AD) is a rare condition. The patient’s condition can develop swiftly and deteriorate rapidly leading to multiple organ failure causing metabolic acidosis and delayed diagnosis.
A 56-year-old man, a cigarette smoker, with a known history of hypertension, gout and kidney disease, presented with cardiac arrest. According to a friend, he had been receiving medication from a private clinic and had been experiencing exertion dyspnea for several months. On admission day, he complained of chest pains when lying down, breathing was hard and he fell unconscious. It took about 30 minutes to transfer the patient, and he stopped breathing on arrival at the hospital. The initial electrocardiography (ECG) showed asystole and cardiopulmonary resuscitation was promptly started. After two doses of intravenous adrenaline, the sinus rhythm returned and BP was 150/90 mmHg. A physical examination showed an overweight man with a coma score of E1M4VT. The pupils were 3mm dilated and sluggishly reacted to light and were slightly deviated to the left. A computed tomography (CT) of the brain showed generalized brain atrophy, and a probable small lacuna infarct. In addition, non-obstructive atherosclerotic changes of intra-cranial arteries were noted with no explainable causes of coma. He had Kussmal breathing with rhonchi, crepitation in both lungs, and a bronchodilator and furosemide were given. All extremities were cold and femoral pulses were not palpable. The chest film revealed a cardiomegaly with bilateral pulmonary edema. The arterial blood gas showed metabolic acidosis (pH 7.32, pCO2 33, pO2 85 mmHg, HCO3 17 mmol/L) and sodium bicarbonate was given. The post-resuscitation ECG revealed a sinus rhythm with a complete right bundle branch block (RBBB), ST elevation in the aVR, J point ST depression in V2-4 and a QT segment of 400 msec (see Figure 1). The echocardiogram showed hypokinesia of the mid-distal septum, with impaired left ventricular systolic function and an ejection fraction of 0.35. The cardiac TnT was elevated at 45ng/L. These findings suggested acute coronary syndrome from the proximal left anterior descending coronary artery, so the patient was referred for an emergent coronary angiography. Informed consent was obtained by relatives.
It was found that both common iliac arteries were occluded, so a coronary angiogram was performed via the rightbrachial artery. The left coronary angiogram showed an unobstructed left main trunk, left anterior descending (LAD) artery, circumflex (Cx) artery which supplied the postero-descending artery (PDA), (see Figure 2B).
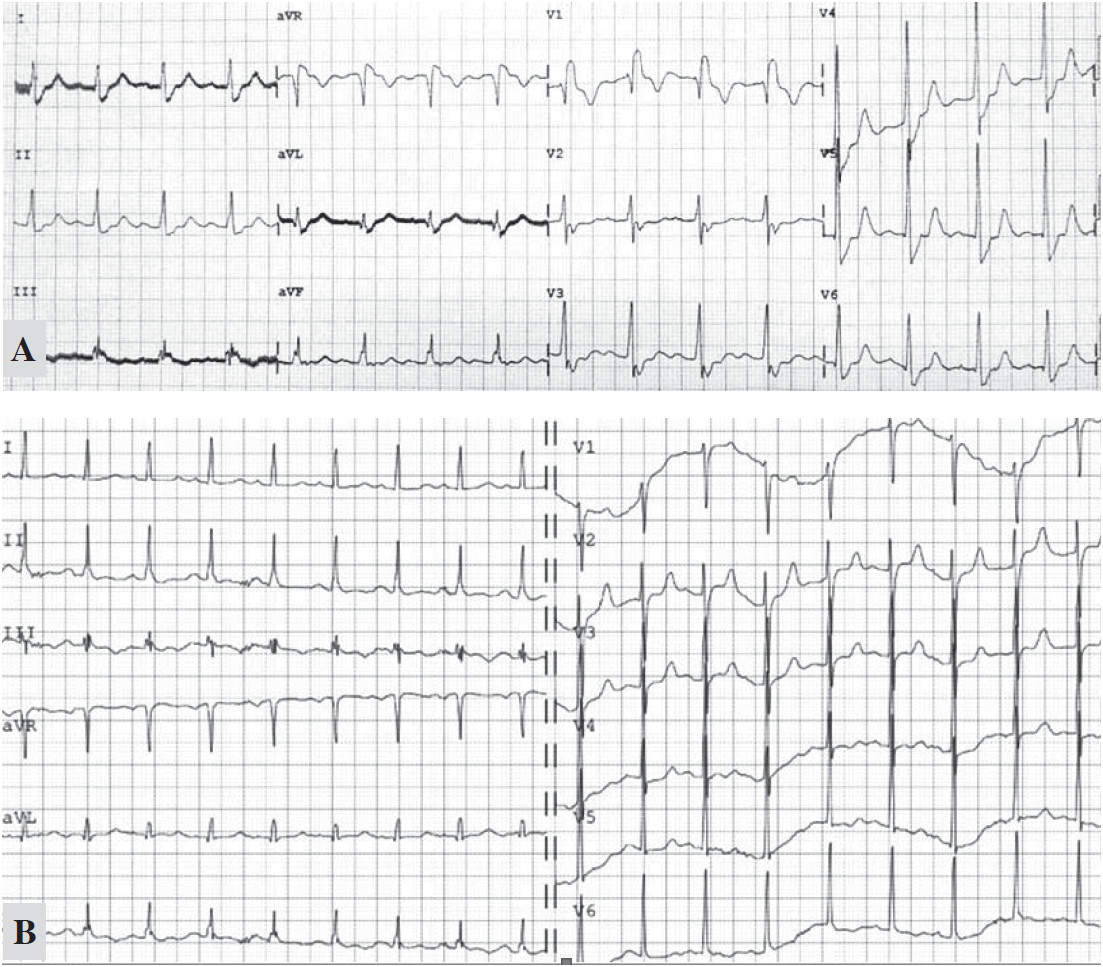
Figure 1A: Post-arrest ECG showed a sinus rhythm rate of 100/min, complete RBBB, J point ST depression in V2-4, ST elevation in leads aVR and lead III.
1B: the RBBB pattern disappeared on the following day.
We could not engage the non-dominant right coronary artery (RCA). An aortogram was performed by hand injection and revealed a totally occluded abdominal aorta below the renal arteries (Figure 2C). Both renal arteries were unobstructed. The aortic CAT scan showed an unobstructed RCA, multiple penetrating ulcers (PAU) from the aortic arch to the abdominal aorta with an intramural hematoma (Figure 3A-C, 4A). There were large intraluminal thrombi obstructing the infra-renal aorta (Figure 3D, 4B) and the inferior mesenteric and bilateral common iliac arteries. There was minor collateral supply to the right common, external, internal iliac arteries and the distal part of the left external iliac artery.
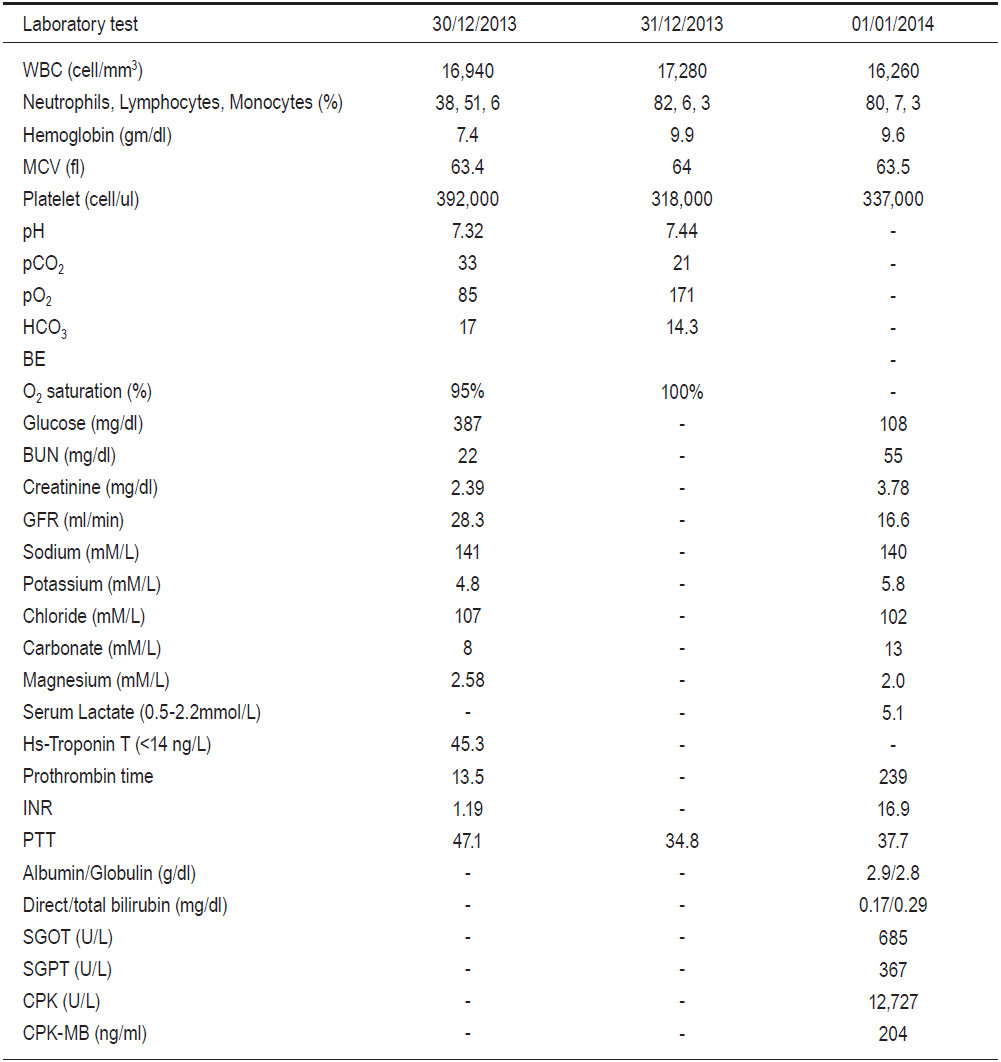
Heparin, anti-platelet medication and hemodialysis were started but the patient remained in a coma with severe metabolic acidosis. The blood ketone was negative and the serum lactate was rising (Table 1). The RBBB ECG pattern disappeared the next day (see Figure 1B). After 24 hours, his condition deteriorated and bluish feet were noted (Figure 5). Vascular surgeons were consulted and it was concluded that it was too late to save him. The serum creatinine rose from 2.39 to 3.78mg/dl without urine output. The liver enzyme and creatinine phosphate were elevated (Table 1). The patient passed away 48 hours after admission from multi-organ failure and uncorrected metabolic acidosis. An autopsy was not authorized by family members.
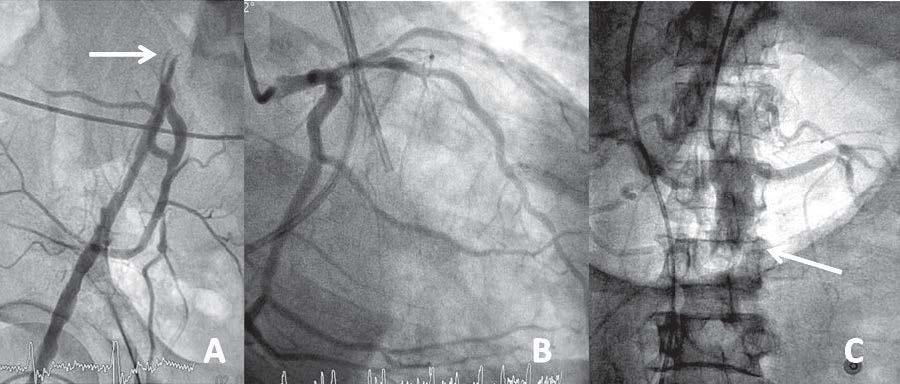
Figure 2: An occluded iliac artery (A), non-obstructive left dominant coronary angiogram (B) and occluded infra-renal aorta (C).
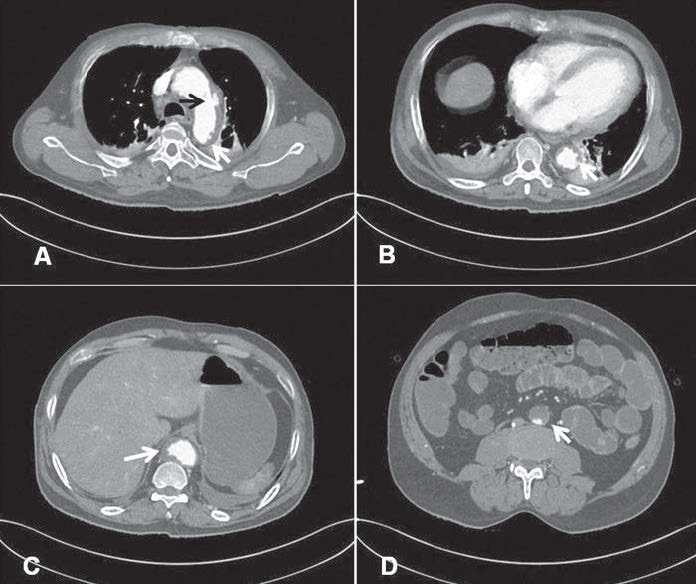
Figure 3: CT shows a large penetrating aortic ulcer (PAU) at the lateral wall of the aortic arch (A, black arrow),multiple small PAU along the abdominal aorta with intramural and mural thrombus (white arrow, A-C). The inferior renal abdominal aorta is occluded with thrombus. Bilateral pleural effusion and atelectasis are also noted (Figure 3A-B).
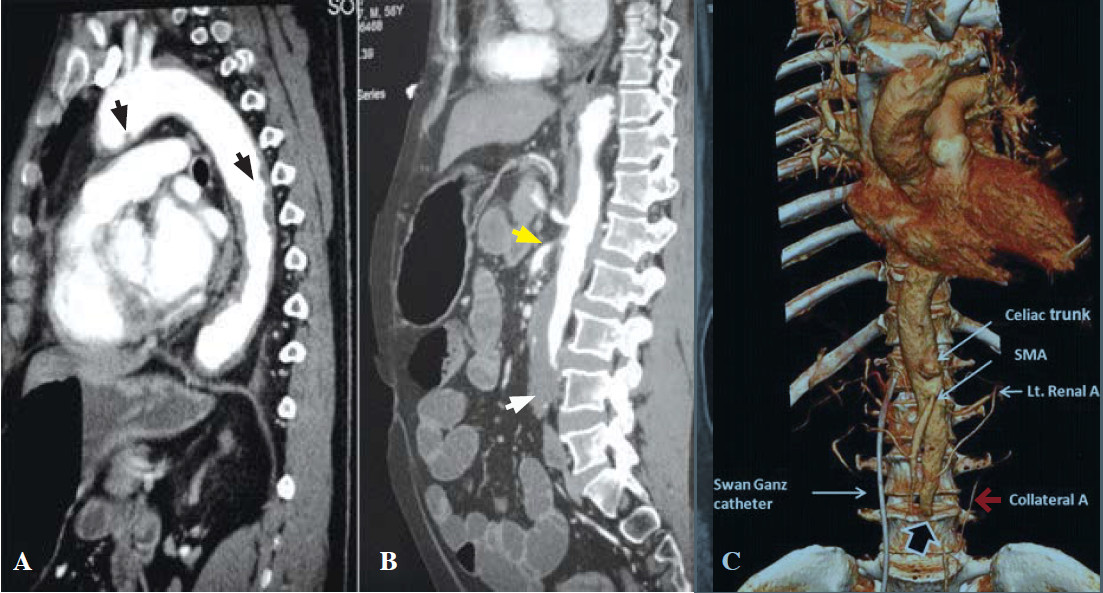
Figure 4: The sagittal CT image shows multiple aortic ulcers (black arrow, A) extending from the arch along the descending aorta and mural thrombi occluded distal abdominal aorta (white arrow, B). The inferior mesentery is occluded. The celiac artery is patent; the superior mesenteric artery is marked as narrow at its origin (yellow arrow, B). The volume-rendered CT image (4C) shows an abdominal aortic occlusion (black arrow) with minimal collateral circulation from the retroperitoneal artery (red arrow, C).
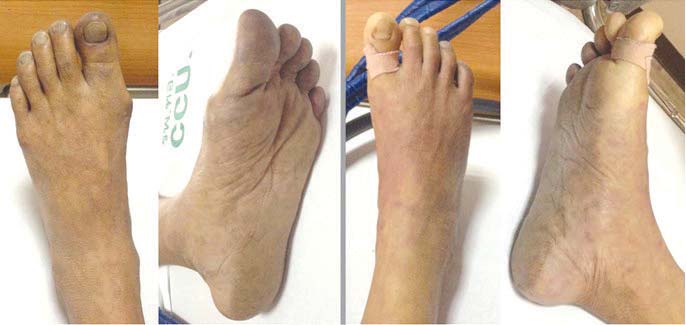
Figure 5: After 24 hours, the patient presented bluish feet caused by vascular occlusion.
Table 1: Laboratory tests from day 1 to day 3.
Acute aortic syndrome
Acute aortic syndrome is a highly fatal aortic disease comprising aortic dissection (AD), intramural hematoma (IMH) and penetrating aortic ulcer (PAU1). AD resulted from a tear in the intima which resulted in blood entering the media, creating a false lumen and mal-perfusion. Unlike intramural thrombus, IMH is located inside the intima and is caused either by spontaneous bleeding of the vasa vasorum in the aortic media or by a PAU.1 PAU is an ulcerated atheromatous plaque that extends from the intima into the aortic media2. The pulsatile blood flow in the wall then creates a medial hemorrhage which causes IMH.1,3 The propagating IMH along the aortic media further weakens the aortic wall leading to AD4, an aortic rupture1 or an inwardly disrupted intima causing a communicating AD.5
Causes of cardiac arrest
Our patient experienced chest pain before collapsing. The cause of asystolic arrest remains unclear. The potassium concentration was normal and there was no significant coronary artery stenosis, no pericardial tamponade and no other explainable cerebral cause identified from the brain scan. A subsequent ECG showed that the RBBB pattern had disappeared (see Figure 1B). His microcytic anemia was caused by a combination of iron deficiency and hemo- globin E trait. There was no evidence of blood loss during admission and the serial hematocrit was stable.
His main CT findings were a large PAU in the lateral wall of the aortic arch (Figure 3A), multiple small PAUs along the descending and abdominal aorta (Figure 3B-C) with diffuse IMH and intramural thrombus occluding the infra-renal abdominal aorta, the inferior mesenteric and the bilateral common iliac arteries. Minor collater- al supply (Figure 4C) suggested an acute thrombosis on top of chronic atheromatous disease at a relatively young age. Although PAU could be an incidental finding, in the Mayo clinic study, most PAU cases (75%) were symptomatic, mostly from back pain, and 30% had pleural effusion.6 The majority of cases in the Mayo clinic study had a history of hypertension (92%) and tobacco smoking (77%).6 Our case also shared these characteristics. Although the CT of chest showed a small pleural effu- sion and atelectasis (Figure 3B), these findings could also have occurred after cardiac arrest. On very rare occasions, PAU patients present with hemoptysis from an oozing hematoma located outside the aorta.7 It is known that PAU and IMH are not benign conditions. Both of these conditions are predominantly located in the descending aorta, associated with hypertension and are a potential cause of AD.1,8-10 Evangelista and colleagues followed 68 IMH cases for 45 months and found that most IMH cases (54%) progressively worsened to become either true (30%) or pseudo-aortic aneurysms (24%). Classic AD and spontaneous regression were observed in 8% and 34% of cases respectively.11 Although our case had several features for developing AD, i.e. long-standing hypertension, smoking, abrupt pain, pulse deficiency and kidney failure, we could not find definitive evidence of either AD or any aneurysm in the multi-plan CT images.
Metabolic acidosis and aorto-iliac occlusion
Most successfully resuscitated cardiac arrest victims do not have persisting metabolic acidosis after spontaneous circulation has been restored. In this case, severe metabolic acidosis was detected throughout and the blood ketone was negative. Table 1 shows increasing levels of serum lactate, liver and muscle enzymes. After 24 hours, ischemic feet became apparent (Figure 5). In 1965, Johnstone and colleagues reported metabolic changes after infra-renal aorto-iliac clamping in six patients who had received reconstructive surgery for aorto-iliac disease. There was a profound fall in oxygen tension in lower limbs causing anaerobic production of lactate and metabolic acidosis.12 In 1993, Whalley and colleagues reported the same findings during the application and removal of aortic cross clamps of the infra-renal aorta in 20 patients who had undergone aneurysmal repairs or procedures to reconstruct occlusive arteries.13 It was noted that the mixed venous blood lactate concentration positively correlated with the time of aortic cross clamp (r = 0.717, p = 0.0297) emphasizing the role of collateral circulation in the development of metabolic acidosis during aortic surgery. Therefore, it is quite possible that the hypo-perfusion stage from prolonged aorto-iliac occlusion caused an over- production of lactic acid and severe metabolic acidosis in our case. In addition, Yamamoto and colleagues reported four patients with acute aortic occlusion and concomitant internal iliac occlusion who had undergone bypass surgery or thrombectomy. All of them were elderly (ages ranged from 63 to 82 years) and presented with lower limb weakness and ischemia. Despite thrombectomy and axillo-bifemoral bypass operations, two patients (age 72 and 85 years) had insufficient re-perfusion of the bilateral internal iliac arteries and died on day three (mortality rate of 50%) from hyperkalemia and multi-organ failure14. An autopsy of one of the cases showed patent superior mesenteric and celiac arteries but occluded bilateral iliac arteries with severe intestinal ischemia. Elevated CPK and myoglobinuria were observed in all patients but this was more prominent in the fatal cases.14 The other possible contributing factor in this case was thrombo-embolism from the athero-thrombotic aorta to the superior mesenteric or celiac arteries. Abdominal distension and leukocytosis strongly supported the manifestation of acute ischemic bowel.15 Unfortunately, these findings were only detected after 24 hours when his condition had already worsened considerably. Liver and kidney failure were noted with a prolonged prothrombin time of 239 seconds (INR of 36.9), an elevated SGOT, a SGPT of 685,367U/L respectively and a creatinine increase from 2.39 to 3.78mg/dl (Table 1). Rhabdomyolysis occurred with CPK of 12,727U/L. Cause of death was severe acidosis and multi-organ failure.
We reported on a relatively young man who presented with cardiac arrest, acute aortic syndrome (PAU and IMH) and severe metabolic acidosis. Initial ECG mimicked anterior myocardial ischemia but no significant coronary artery lesion was found. CT scan revealed multiple penetrating aortic ulcerations, intramural hematoma, mural thrombus occluding infra-renal aorta, inferior mesenteric and bilateral common iliac arteries causing lower limbs and possible intestinal ischemia. The patient’s condition rapidly deteriorated and the cause of death was severe metabolic acidosis and multi-organ failure. This was a very high risk patient presenting a fatal aorto-iliac occlusion case which required early diagnosis and inter- vention.