Carotid artery disease is one of the main causes of stroke, apart from other cardiac causes or disease of the brain itself. About 80% of all strokes are ischemic and approximately 25-50% of these are caused by an unstable carotid artery plaque.1 The risk factors for carotid stenosis are similar to those for athero- sclerosis and include hypertension, diabetes, cigarette smoking and dyslipidemia. Prevention of stroke caused by carotid bifurcation stenosis can be achieved by accurate identification and evaluation of patients at risk. Pathophysiology of carotid atherosclerosis is similar to that in other vascular beds. However, atherosclerosis in the carotid artery is usually unifocal, and 90% of lesions are located within 2 cm. of the internal carotid artery (ICA) origin. Extracranial internal carotid artery stenosis accounts for 15 to 20% of ischemic strokes, depending on the population studied. The degree of carotid stenosis is associated with the degree of stroke risk. Carotid ath- erosclerosis can produce retinal and cerebral symptoms by way of 1 of 2 major mechanisms. progressive carotid stenosis leading to insitu occlusion and hypoperfusion (less common), or intracranial arterial occlusion resulting from embolization (more common). Embolism from unstable plaque is the major mechanism of stroke in carotid atherosclerosis.2,3 Stroke is more likely to be due to embolism rather than hypoperfusion even in patients with >70% carotid stenosis, and the risk of stroke may be lower in patients with >90% stenosis, due to post-stenotic narrowing in the distal ICA, which reduces flow and risk of embolism. Patients present- ing with carotid distribution cerebral ischemia should be thoroughly evaluated for treatable causes, including sources of emboli from the carotid arteries, heart, and aortic arch. Patients with or without carotid stenosis may also develop symptomatic cerebral hypoperfusion from systemic causes.
A carotid bruit is the most common clinical finding, although its positive predictive value is only about 60 to 70 percent. A carotid bruit is identified in 4% to 5% of patients age 45 to 80 years, and should be heard in the majority of patients with carotid stenosis greater than or equal to 75%. However, a bruit may be absent if there is slow flow through a severe stenosis.
Muluk et al. in 1999 studied serial duplex scans in 1701 carotid arteries in 1004 asymptomatic patients over a 10-year period.4 The risk of progression of ICA stenosis increased steadily with time (annualized risk of progression, 9.3%). With multivariate modeling, the four most important variables that affected the progression were baseline ipsilateral internal carotid artery (ICA) stenosis ≥50%, baseline ipsilateral external carotid artery (ECA) stenosis ≥50%, baseline contralateral ICA stenosis ≥50%, and systolic pressure more than 160 mm Hg. Ipsilateral neurologic ischemic events (stroke/transient ischemic attack) occurred in association with 14% of the carotid arteries that were studied. The progression of ICA stenosis correlated with these events, but baseline ICA stenosis was not a significant predictor.
Three treatments for this problem are (1) medical therapy (2) carotid stenting (CS) and (3) carotid endar- terectomy (CE). CE, surgical removal of the carotid atherosclerotic plaque, has formed the mainstay of surgical treatment. Endovascular angioplasty (with/ without stenting) for carotid stenosis, less invasive technique for carotid artery revascularization, has been proposed as a viable alternative to carotid endarterectomy. Current guidelines for CE in symptomatic carotid stenosis are based on two randomized; controlled trials,the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the European Carotid Surgery Trial (ECST), both from the 1990s.5-7 In both trials, the degree of stenosis, estimated from a catheter angiogram, was the major criterion for recommending CE. According to these trials, the benefit from CE is greatest in patients with 70-99% stenosis with a 5-year absolute risk reduction of 15.3%, less, only 7.8% in those with 50-70% stenosis and minimal in those with <50% stenosis. Since then there were several clinical trials published.
The objectives of this review are (1) to provide evidences of benefit/risk of each treatment and (2) to create recommendation for patients.
Five different aspects should be considered as to the treatment of patients with carotid disease:
Traditional imaging methods of carotid artery disease include angiography, duplex ultrasound and computed tomography angiography (CTA). These methods mainly focus on anatomic features of the plaque; however, some techniques are also able to detect morphologic characteristics of plaque vulnerability such as ulceration, a large lipid or necrotic core and a thin fibrous cap. Angiography was the gold standard in the NASCET and ECST to determine degree of stenosis. Duplex ultraso- nography and CTA are also being used to determine the degree of stenosis in carotid artery disease. With regard to plaque morphology, several studies have compared the imaging results to histopathological findings as the gold standard. Angiography was able to detect ulceration with a sensitivity and specificity of approximately 45% and 75%, respectively.13, 14 CTA has also shown to identify plaque ulceration, calcification and lipid cores with an overall agreement of about 75% between CTA findings and histology.15, 16 Among the current clinically available imaging modalities, MRI seems the most accurate method to image plaque morphology in carotid artery disease. Various advanced imaging methods are available, such as high-resolution magnetic resonance imaging, single photon emission computed tomography (SPECT), positron emission tomography (PET) and near-infrared fluorescence. Radionuclide and fluorescent tracers that identify inflammation, apoptosis and proteolysis, are promising.17 A combination of activity of molecular processes and detailed anatomic information can be obtained, providing a powerful tool in the identification of the vulnerable plaque. With these developments, we are entering a new era of imaging techniques in the selection of patients for carotid surgery.
In routine clinical practice, the indication to treat using invasive techniques is usually based on 1 and 2, while the choice between carotid endarterectomy (CE) and carotid artery stenting (CS) is mainly based on 3, 4 and 5.
The following are definitions of the classification of the evidence (Class I-IV) and classification of recom- mendation (A, B, C and U) used in this article.
Classification of Evidence
Class I: Prospective, randomized, controlled clinical trial (RCT)with masked outcome assessment, in a representative population. The following are required:
a.Primary outcome(s) clearly defined.
b.Exclusion/inclusion criteria clearly defined.
c.Adequate accounting for dropouts and crossovers with numbers sufficiently low to have minimal potential for bias.
d.Relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences.
Class II: Prospective matched group cohort study in a representative population with masked outcome assess- ment that meets a-d above OR a RCT in a representative population that lacks one criterion a-d.
Class III: All other controlled trials (including wellde- fined natural history controls or patients serving as own controls) in a representative population, where outcome is independently assessed, or independently derived by objective outcome measurement.
Class IV: Evidence from uncontrolled studies, case series, case reports, or expert opinion.
Classification of Recommendation
A = Established as effective, ineffective, or harmful for the given condition in the specified population. (Level A rating requires at least two consistent Class I studies.)
B = Probably effective, ineffective, or harmful for the given condition in the specified population. (Level B rating requires at least one Class I study or at least two consistent Class II studies.)
C = Possibly effective, ineffective, or harmful for the given condition in the specified population. (Level C rating requires at least one Class II study or two consis- tent Class III studies.)
U = Data inadequate or conflicting; given current knowledge, treatment is unproven.
Atherosclerosis is a progressive systemic disease with strong relationships in the prevalence of plaques in different sites of arterial system. Carotid intima-media thickness (IMT) is a validated measure of atherosclerosis burden and is most reproducibly evaluated in the far wall of the distal common carotid artery18 moreover, carotid atherosclerosis is a risk factor for several chronic diseases, including coronary artery disease19 and stroke20. Carotid atherosclerotic plaque rupture is thought to cause transient ischemic attack and ischemic stroke. Patho- logical hallmarks of these plaques have been identified through observational studies.
Statins showed anti-atherosclerosis through pleiotropic effects.21, 22 Statin significantly reduces the progression of early, preintrusive atherosclerosis. A trend for reduction in carotid IMT was shown after only 6 months of therapy.23 Aggressive statins were more −0.063 mm/y of reduction in annual progression of carotid atherosclerosisthan con- ventional statins therapy.24 The latest recommendations for primary prevention of stroke from the European Stroke Organization are that blood cholesterol should be checked regularly; high cholesterol (e.g., LDL cholesterol >3.9 mmol/L [150 mg/dL]) should be managed with lifestyle modification (class IV, level C) and a statin (class I, level A). In secondary prevention of stroke, statin therapy is recommended for patients with non-cardioembolic stroke (class I, level A).25 In secondary prevention of stroke, evidence-based data from the only available trial were obtained with a high dose of atorvastatin (80 mg per day26); post-hoc analysis of the subgroup of patients with Heart Protection Study (HPS) who had a previous stroke found no effect on stroke recurrence with simvastatin 20–40 mg per day.27 An analysis from the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial showed that lowering of LDL cholesterol concentrations to less than 1.8 mmol/L compared with more than 2.6 mmol/L (70 vs 100 mg/dL) was followed by a 28% reduction in relative risk for stroke.28 This result was obtained post-hoc and, therefore, is hypothesis generating. The next step would be to show that, in patients with a stroke or transient ischaemic attack, an LDLcholesterol concentration of less than 1.8 mmol/L (70 mg/dL) is associated with a lower incidence of recurrent stroke or other major vascular events than is a concentration of less than 2.6 mmol/L (100 mg/dL).29
β-blocker can reduce the rate of carotid intima media thickness progression in clinically healthy, symptomfree subjects with carotid plaque30, the results suggest that the autonomic nervous system may be an important role in atherosclerosis development in otherwise healthy people with carotid plaque. Secondary stroke prevention after transient ischemic stroke or minor stroke is of major importance in order to avoid recurrent cerebrovascular events and decrease morbidity and mortality.
For patients with non-cardioembolic stroke, antiplatelet agents are the treatment of choice. Aspirin (81 to 325 mg) plus extended-release dipyridamole and clopidogrel are more effective than aspirin and should be used in patients with a high risk of recurrent stroke.31 Oral anticoagulations are highly effective in patients with a cardiac source of embolism. Medical therapy alone is preferred for patients in whom the risk of revascularization outweighs its benefits, including patients who are at low risk for stroke with medical therapy (symptomatic stenosis less than 50%, asymptomatic stenosis less than 60%), and those with a highrisk of procedure-related stroke or death due to clinical or technical factors. Patients with transient ischemic attack (TIA) / minor stroke should be seen as soon as possible in dedicated centers that offer single visit imaging. All patients should start taking their risk factor medications as soon as possible and patients with a 50 – 99% ipsilateral internal carotid artery stenosis should be transferred to the Vascular clinic for further managements.
Carotid angioplasty and stenting (CS) has steadily developed over the preceding decade. The main advantages of CS over CE are that the procedure is less invasive, performed under local anaesthesia, and is less influenced by the co-morbidities of the patient, while the outcomes are determined mainly by anatomical or procedural variables.32-34 The disadvantages of CS are (1) it is not suitable if there is a contrast allergy, severe aortic arch atheroma, highly tortuous arteries or lumenthrombus (2) femoral artery puncture is required, which may cause a cutaneous or femoral nerve injury, and a wound haema- toma which may become infected or compress vital groin structures (3) higher total procedural costs due to more expensive devices used for endovascular treatment (4) it may cause a stroke, as a result of arterial dissection, late embolization of thrombus on damaged plaque, hypo- tension (carotid sinus stimulation), aneurysm formation, or arterial puncture (5) uncertain durability over many years in preventing ipsilateral carotid ischemic stroke. Although no randomized study has compared carotid angioplasty vs. stenting, virtually all endovascular carotid procedures currently performed are stentbased. Carotid stents are self-expanding and the vast majority of them are made of nitinol.
Patients undergoing CS are commonly pre-treated with aspirin and clopidogrel. Aspirin is continued lifelong and clopidogrel given for at least 1 month after the procedure. The concept of dual antiplatelet therapy came from the coronary experience and was immediately embraced by part of the interventional community also for the endovascular treatment of the carotid arteries. Small randomized trials comparing single with double antiplatelet therapy for CS followed but had to be prematurely terminated due to high stent thrombosis and neurological event rates in the aspirinonly group.35
The first randomized trial comparing endovascular and surgical treatments for carotid stenosis patients, CAVATAS (Carotid and Vertebral Artery Transluminal Angioplasty Study)36, which was published in 2001, included 504 patients enrolled between 1992 and 1997 and was designed to compare balloon angioplasty alone versus CE. Stents, when they became available, were incorporated as well but only accounted for 26% of cases. The CAVATAS trial demonstrated no statistically significant difference between endovascular and surgical treatment in the rate of disabling stroke or death within 30 days (6.4% CS vs. 5.9% CE) and no significant difference in the 3-year ipsilateral stroke rate. These early encouraging results generated a great deal of interest in CS, and further studies were undertaken. The long-term effectiveness of endovascular treatment of this trial reported in 2009.37 Patients who were randomly assigned in CAVATAS and completed treatment for carotid stenosis (200 patients had endovascular treatment and 213 patients had CE) had prospective clinical follow-up at a median of 5 years and carotid duplex ultrasound at a median of 4 years. Severe carotid restenosis (≥70%) or occlusion occurred significantly more often in patients in the CS than in patients in the CE (adjusted hazard ratio [HR] 3.17, 95% CI 1.89 – 5.32; p<0·0001). The estimated 5-year incidence of restenosis was 30.7% in the CS and 10.5% in the CE. Patients in the endovascular arm who were treated with a stent (n=50) had a significantly lower risk of developing restenosis of 70% or greater compared with those treated with balloon angioplasty alone (n=145; HR 0.43, 0.19–0.97; p=0·04). Current smoking or a history of smoking was a predictor of restenosis of 70% or more (2.32, 1.19–4.54; p=0·01) and the early finding of moderate stenosis (50 – 69%) up to 60 days after treatment was associated with the risk of progression to restenosis of 70% or more (3.76, 1.88 – 7.52; p=0·0002). There were more patients with non-perioperative ipsilateral stroke or transient ischaemic attack (HR 1.29, 95% CI – 2.14) and more patients with non-perioperative ipsilateral stroke (1.22, 0.59 – 2.54) in the endovascular arm than there were in the endarterectomy arm during follow-up, although these differences were not statistically significant. The increase in events in the endovascular arm might be partly explained by the high incidence of restenosis after endovascular treatment.
Hassan Murad M., et al. in 2008 performed systemic review and meta-analysis compared CE vs CS for carotid artery stenosis.38 Ten randomized controlled trials (RCTs) with 3182 participants proved eligible, provided low to moderate quality evidence. At 30 days and compared with CE, CS was associated with a nonsignificant reduction in the risk of death in five studies (RR, 0.61; 95% CI, 0.27-1.37; I2 = 0%); a nonsignificant reduction in the risk of nonfatal MI in 3 studies (RR, 0.43; 95% CI, 0.17-1.11; I2 = 0%); and a nonsignificant increase in the risk of any stroke in 5 studies (RR, 1.29; 95% CI, 0.73-2.26; I2 = 40%). When only major and disabling strokes were included in the analysis, a similar nonsignificant increase in the risk of stroke was noted in patients who received CS in 4 studies (RR, 1.06; 95% CI, 0.32-3.52; I2 = 45%). When only Q-wave myocardial infarctions (MIs) we reincluded in analysis, data were very limited and precluded meaningful analysis (1 Q-wave MI in the CS group vs 4 in the CE group). These results came from only two trials, because the other trials did not differentiate between Q and non-Q wave MI. These analysis limitations are blind of data in each study and half of the trials were stopped early and yielded imprecise results on the outcome of stroke, which is the main outcome these two procedures are primarily intended to prevent. Finally, both procedures appear equivalent on their effects on death and nonfatal MI; the difference in risk of strokes between procedures remains inconclusive, with a trend toward superiority favoring CE.
The American College of Cardiology Foundation (ACCF) Task Force on Clinical Expert Consensus Documents (CECD), and was cosponsored by the Society for Cardiovascular Angiography and Interventions (SCAI), the Society for Vascular Medicine and Biology (SVMB), the Society of Interventional Radiology (SIR), and the American Society of Interventional & Therapeutic Neuroradiology (ASITN) published a guideline for carotid artery stenting (CAS) in 2007.39 The European Society for Vascular Surgery (ESVS) again brought together a group of experts in the field of carotid artery disease to produce updated guidelines for the invasive treatment of carotid disease in 2009.40
The available level I evidence suggests that for symp- tomatic patients, surgery is currently the best option [A]. Mid-term stroke prevention after successful CS is similar to CE [A]. CS should be offered to symptomatic patients, if they are at high risk for CE, in highvolume centers with documented low peri-procedural stroke and death rates or inside an RCT [C].
CS is a reasonable alternative to CE, particularly in patients at high risk for CE. The concept of a high-risk patient is very controversial. It appears that when patients meet North American Symptomatic Carotid Endarterectomy Trial (NASCET)/the Asymptomatic Carotid Atherosclerosis Study (ACAS) exclusion criteria,they are automatically defined as high risk.
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy trial (SAPPHIRE) is the only randomized trial comparing CS and CE performed with the systematic use of Embolic Protection Devices (EPDs).41 The trial included symptomatic and asymptomatic patients at high risk for surgery and wasdesigned to prove the noninferiority of the endovascularapproach. According to the SAPPHIRE trial, a high-risk patient with medical co-morbidities has one of the following features:
Barbato J. E., et al. in 2008 performed a prospective, randomized, single-center study of CS with or without a distal cerebral protection filter.42 A 1:1 scheme was used to randomize 36 carotid artery stenting procedures in 35 patients. Diffusion-weighted magnetic resonance imaging 24 hours after stenting was used to assess the occurrence of new embolic lesions. Four strokes occurred (11%), two in each group, in patients aged 75, 80, 82, and 84 years.
They concluded that the use of filters during CS provided no demonstrable reduction of microemboli. However Garg N., et al. in 2009 performed a random effects meta-analysis of studies with concurrently reported data on protected and unprotected CS.43 Initial database queries resulted in 2485 articles, of which 134 were included in the final analyses (12,263 protected CAS patients and 11,198 unprotected CS patients). Using pooled analysis of all 134 reports, the relative risk (RR) for stroke was 0.62 (95% CI 0.54 to 0.72) in favor of protected CS. Subgroup analysis revealed a significant benefit for protected CS in both symptomatic (RR 0.67; 95% CI 0.52 to 0.56) and asymptomatic (RR 0.61; 95%CI 0.41 to 0.90) patients (p<0.05).
Zarins C. K., et al. in 2009 reported a prospective, nonrandomized comparative cohort study of a broad-risk population of symptomatic and asymptomatic patients with carotid stenosis namely “Carotid revascularization using endarterectomy or stenting systems” (CaRESS).44 There were 397 patients enrolled (254 underwent CE and 143 underwent protected CS). More than 90% of patients had >75% stenosis; two thirds were asymptom- atic. The risk of death or nonfatal stroke 4 years following CS with distal protection is equivalent to CE in a broad category of patients with carotid stenosis. There were no significant differences in stroke or mortality rates between high-risk and nonhigh-risk patients and no differences in outcomes between symptomatic and asymptomatic patients. After 4 years, CS had a 2-fold higher restenosis rate compared to CE. The risk of death/ stroke or death/stroke/MI appears to be higher following CE than CS among patients
Asymptomatic Carotid Surgery Trial-2 (ACST-2) is a randomized clinical trial comparing carotid endarterec- tomy with carotid artery stenting in patients with asymp- tomatic carotid artery stenosis. At least 5000 patients with asymptomatic carotid stenosis are thought to be needed to participate. It will provide important evidence comparing the immediate and long-term safety and efficacy of CE and CS in patients with asymptomatic carotid stenosis.
Generally, treatment for symptomatic carotid stenosis is settled with interventions such as either CS or CE. However treatments for asymptomatic carotid stenosis are controversial. Symptomatic patients with moderate stenosis of the carotid artery (50-69% stenosis), or intraplaque hemorrhage demonstrated by MRI is a good indicator of recurrent ipsilateral stroke and TIA and may be used to improve patient selection for carotid surgery.45
In the Asymptomatic Carotid Surgery Trial (ACST), the annual risk of stroke after CE (0.55%) was much less than the annual risk with the Best Medical Treatment (BMT) alone (1.9%).46 Both the Asymptomatic Carotid Atherosclerosis Study (ACAS) and ACST studies com- pared CE plus BMT versus BMT alone, and both studies demonstrated a decreased risk of stroke by approximately 50% at 5 years.47 The current guideline recommendation Table 1) for asymptomatic patient is to perform CE in asymptomatic men with 48-50
Abbott was one of the first to observe that the annual risk of stroke in medically treated patients has declined significantly over the last 20 years and the latest meta- analysis concludes that non-interventional therapy is the safer option, whilst also being more costeffective.48 A second (smaller) meta-analysis published in 2010 included natural history data from three studies recruiting after 2000 and found that the average annual risk of ipsilateral stroke in 1635 medically treated patients was 0.5%.53 Abbott and others have attributed this decline in stroke risk to improvements in BMT, especially through the use of high dose statins.54, 55 Not surprisingly, this has elicited the inevitable counter- argument, primarily because some studies in Abbott’s meta-analysis included patients with 50–99% as op- posed to 60–99% stenoses.
Since there is a trend of reduction of incidence of stroke over the years after improvement of medical treatments, we need to undertake an adequately powered randomized trial which includes treatment arms for CE, CS and BMT. This should make it possible to test algorithms for identifying ‘high risk for stroke’ subgroups (e.g., transcranial Doppler embolisation, silent infarction on CT, incomplete circle of Willis, computerised plaque morphology, biomarkers).
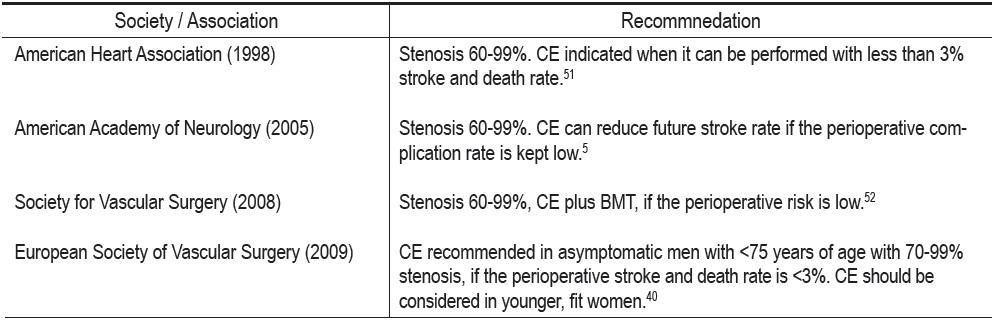
Table 1: Guidelines from various organizations for carotid endarterectomy of asymptomatic carotid stenosis.
The CE is absolutely indicated in symptomatic patients with >70% (NASCET) stenosis [A] and probably with >50% (NASCET) stenosis [A]. The perioperative stroke/death rate should be <6%. CE is contraindicated for symptomatic patients with less than 50% stenosis [A]. CE should be performed within 2 weeks of the patient’s last symptoms [A].
There is still considerable controversy with regard to the role of prophylactic CE in coronary artery bypass grafting (CABG) patients with coexistent carotid artery disease. In many centres around the world, the detection of a carotid stenosis greater than 70% (irrespective of neuro- logical symptom status) will prompt either synchronous or staged CE plus CABG. A 2003 systematic review of 8972 patients undergoing synchronous or staged CEA and CABG identified three studies (99 patients) where in CE was performed immediately prior to off-pump coronary artery bypass grafting (OPCAB) with a reported 30-day death/stroke rate of 1.0%.56, 57 This was considerably less than comparable reported risks for patients undergoing synchronous CE plus On-Pump CABG [30-day death/ stroke 8.7% (95% confidence interval (CI): 7.7–9.8)], staged CE–CABG [30-day death/stroke 6.1% (95% CI: 2.9– 9.3)] and reverse-staged On-Pump CABG–CE [30-day death/stroke rate 7.3% (95% CI: 1.7–12.9)].58
In relation to the peripheral vascular disease, the prevalence of internal carotid artery stenosis of 70% in patients with peripheral vascular disease was 24.7%.59 Age, smoking quantity and a carotid bruit were independent risk factors associated with severe carotid stenosis. Routine duplex screening is recommended in patients with peripheral vascular disease, particularly in male, elderly smokers.
Contraindications to CE are carotid stenosis at surgically inaccessible sites, recurrent stenosis after previous endarterectomy, and stenosis after irradiation. Cervical irradiation is a known risk factor for accelerating carotid stenosis progression. Carmody et al. demonstrated a 22% prevalence of >70% carotid stenosis in patients with previous neck radiotherapy compared with 4% in controls.60 Eighty percent of patients with significant stenosis in the irradiated group were symptomatic. CE in these patients is hindered by previous surgical reconstructions and radiation-induced fibrosis that obliterates the endarterectomy plane and, as a result, is often associated with interposition graft placement. CE in these patients is not associated with a greater risk of stroke; however, a higher incidence of arterial damage, cranial nerve palsy, prosthetic infection, anasto-motic breakdown, restenosis, and an increased rate of wound complications have been reported.61
Octogenarians alone are not a contraindication for CE. Octogenarians undergoing CS had a 3.46-times higher absolute risk of stroke than those undergoing CE. CS in octogenarians using current technology should be avoided in favor of CE or possibly medical management unless a stroke rate of less than 3% can be achieved.62
The incidence of carotid artery stenosis in Thailand is currently unknown; however we estimated it would increase together with the increasing incidence of coronary artery disease in Thailand. The number of patients who underwent CE at the Bangkok Medical Center has increased over the year since K.T. started a CE program.
The following are MRA (Figure 1), endarterectomy specimen (Figure 2 a, b) and CE with vein patch (Figure 3) in one of our patients. This patient recovered well without any complication; however he would face a higher risk of stroke if CS was performed.
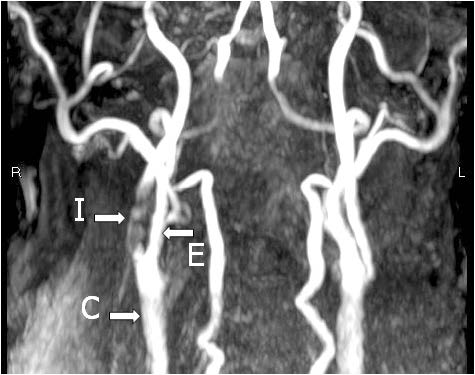
Figure 1: The 3D, Time of Flight (TOF) extracranial MRA: Severe stenosis of the right internal carotid artery (I). E = external carotid artery, C = Common Carotid Artery
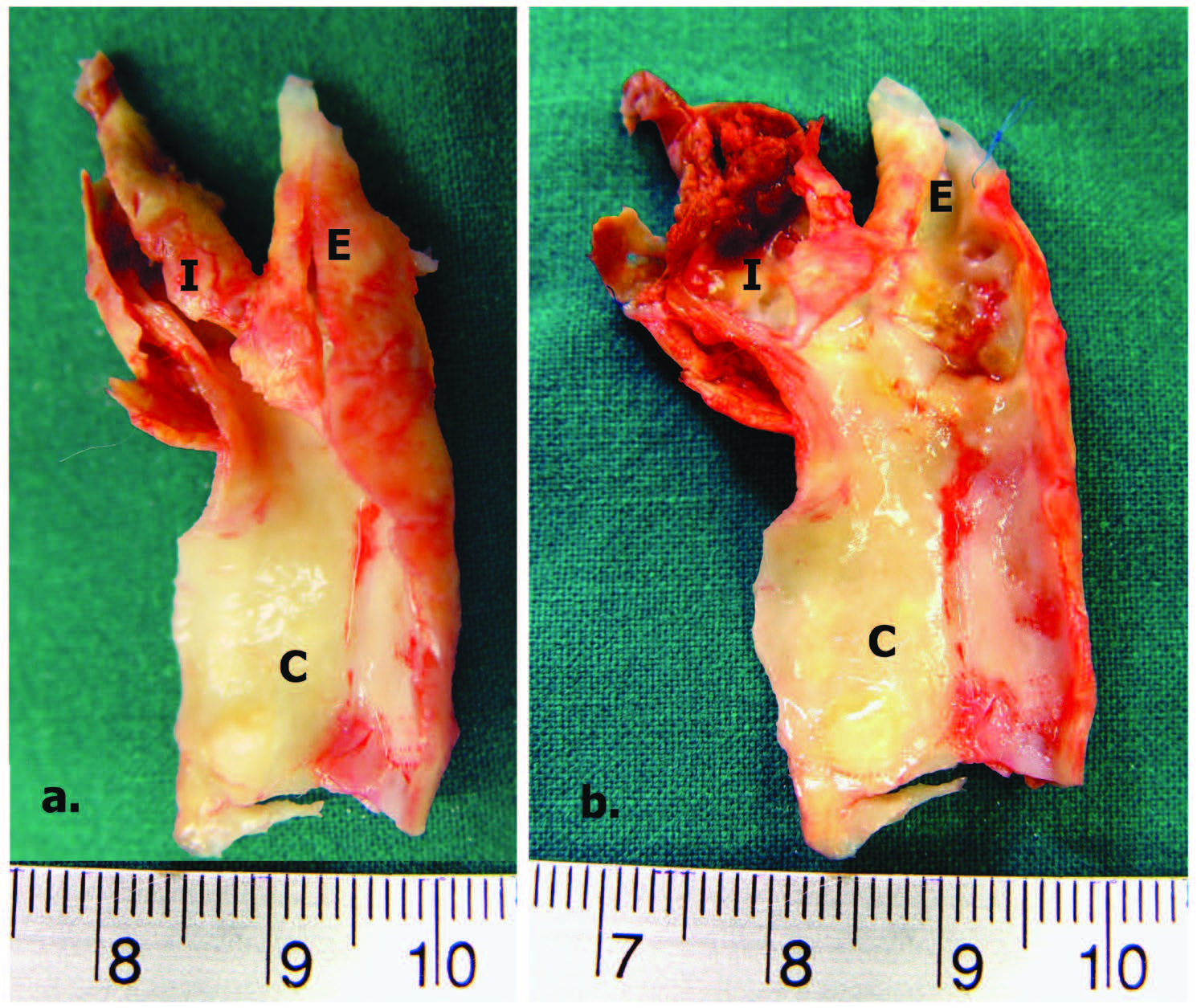
Figure 2: Carotid Endarterectomy Specimens (a) unopened right internal carotid artery (b) opened right internal carotid artery, demonstrated severe stenosis and unstable plaque. I = Internal carotid artery, E = External carotid artery, C = Common Carotid artery
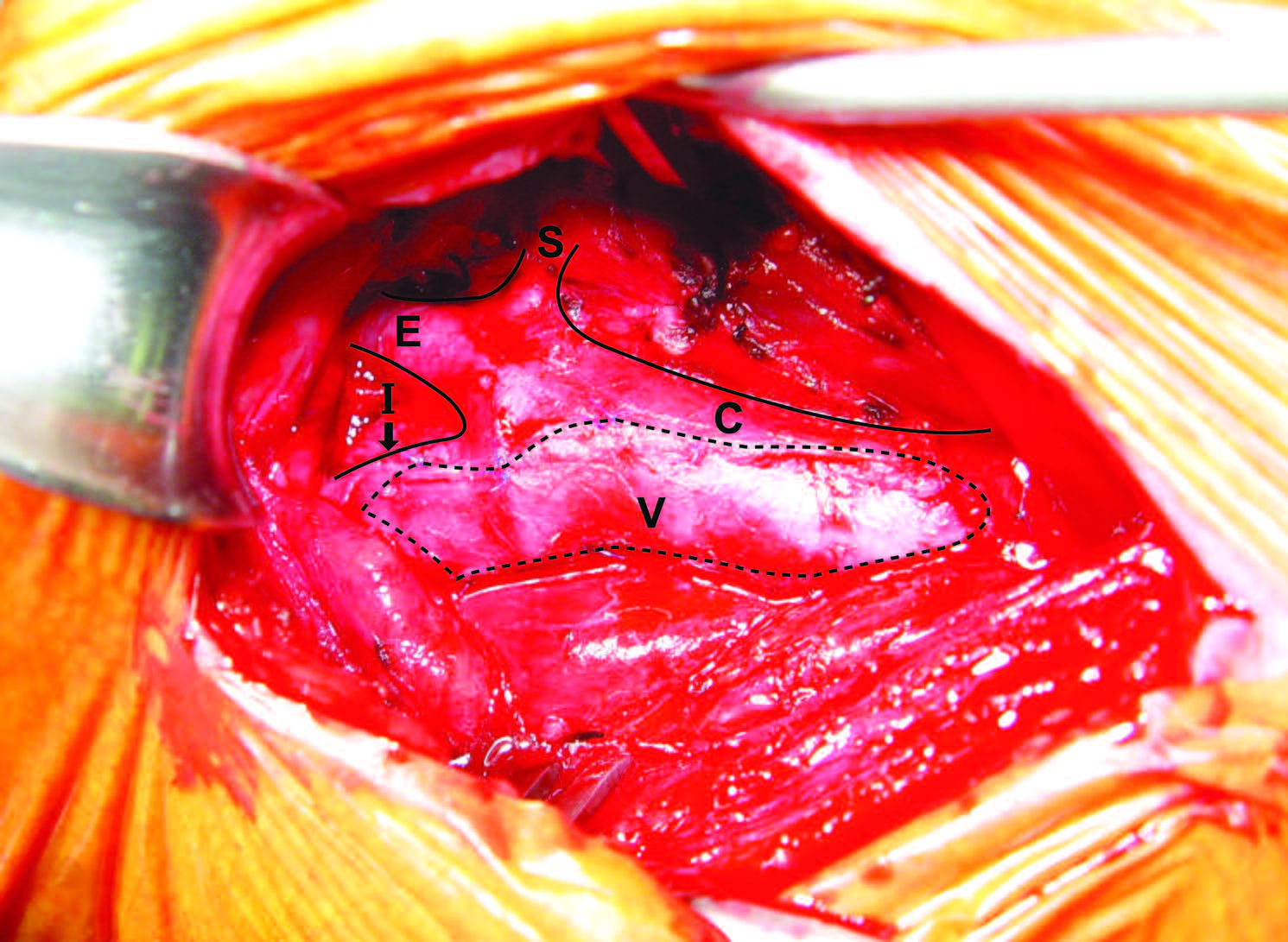
Figure 3: Carotid Endarterectomy with vein patch: the vein patch extended from the right internal carotid artery (I) to the right common carotid artery (C). E = External carotid artery. S = Superior thyroid artery
Intensive medical treatments are absolute indicated in all patients with carotid disease. CE currently remains the first choice of revascularisation therapy for an asymptomatic carotid lesion in most treament centres. For symptomatic patients, CE is much safer than CS, particularly for patients older than 70 years. CS might be considered for patients with limited access to surgery or for difficult technical surgery.