Contrast enhanced spectral mammography (CESM), is a dual energy contrast enhanced digital subtraction mammography. This technique uses the same principle as MRI of the breast, but CESM uses digital mammography instead of magnetic resonance in MRI. It provides dual energy acquisitions through low and high energies in different filters. The subtraction is obtained by emitting a different energy after a complete non-ionic contrast media injection after 2 minutes with breast compression and preprogrammed software without motion artifact. The benefits include better resolution, and ability to evaluate microcalcifications, it is more cost-effective, easier to administer with a shorter time of examination.1
The proposed indications for CESM are as follows:
These situations predominantly include asymmetries, architectural distortions, numerous medium and/or large sized BIRADS 3 lesions and equivocal changes in the appearance of prior surgical or biopsy sites. Contrast enhanced magnetic resonance imaging (CE-MRI) is highly sensitive, but the specificity and negative predictive value are not sufficiently high to preclude biopsy when there are suspicious imaging findings, 67.4% and 85.4%, respectively by Bluemke et al.2 However, in a recent study of 115 patients by Moy Let al,3 the magnetic resonance image (MRI) was performed to evaluate equivocal mammographic findings, following a full diagnostic workup. The study found 100% sensitivity and 92% specificity of MRI. Presumably CESM should have more or less the same results.
Breast MRI, when combined with mammography and a clinical breast exam, has been shown to provide sensitivity of 99% for the preoperative assessment of the local extent of disease in patients with newly diagnosed breast cancer.4 This is compared with sensitivities of 50% for clinical breast exam, 60% for mammography and 83% for ultrasound alone. Breast MRI can detect any additional unsuspected malignancy with in the ipsilateral breast, in 10% to 27% of patients.5-8 When correlated with pathologic specimens, tumour size is more accurate with MRI than mammo-graphy.9,10 Lehman et al. identified otherwise occult tumors in the contralateral breast in 6% of patients with newly diagnosed invasive lobular carcinoma and 3% in patients with invasive ductal carcinoma.11
We found the same findings in CESM, but the data collected in our study is not sufficient to show good statistics. According to our observations, microcalcifications are seen in CESM as normally seen white spots in low energy digital mammography and dark spots in high energy CESM. The CE-MRI cannot show these microcalcifications, which may be the only finding in ductal carcinoma in situ (DCIS). MRI requires an additional mammography to detect microcalcifications, but in one CESM examination, the low energy digital mammography is already provided. We demonstrate 3 different patterns of ultrasound (US) findings where the CESM of each finding is not the same: this is very beneficial in obtaining a more accurate diagnosis.
1. Examples of three patients whose ultrasound (US) shows microlobulate or irregular shaped cystic mass, with internal solid component, the CESM reveals three different findings, indicating different pathologies. The images and final pathological results are presented as follows:
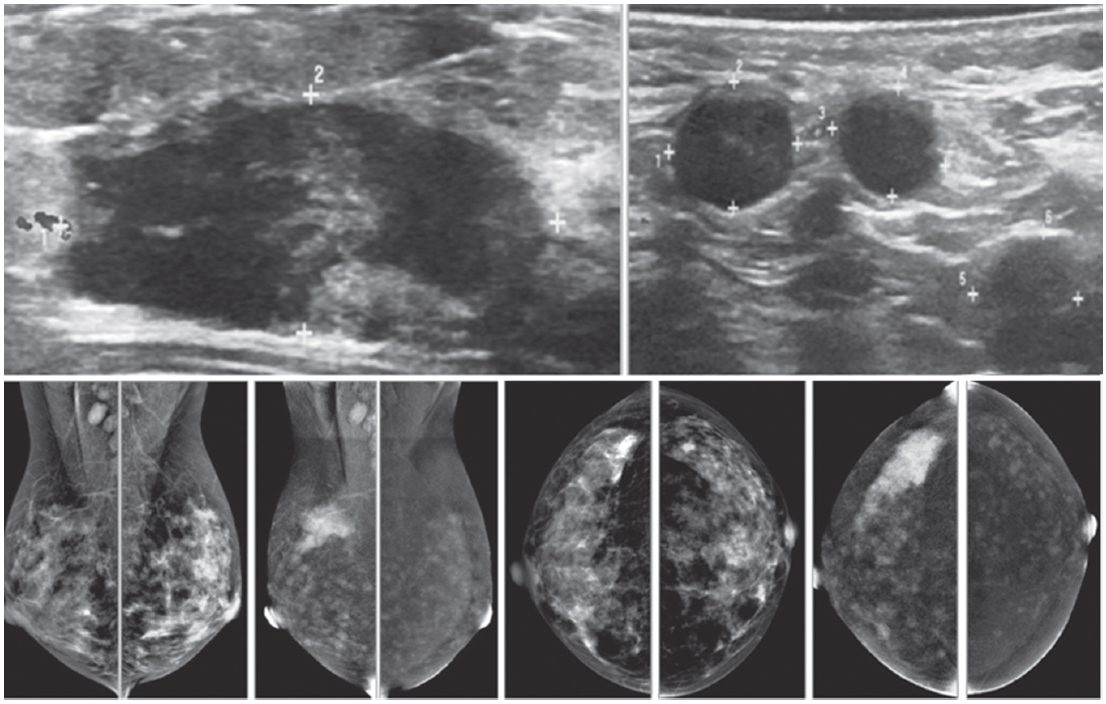
Figure 1A: Female 48-years of age, ultrasound (US) shows a microlobulate, irregular shaped cystic mass, with internal solid component. Multiple enlarged axillaries denote a denopathy with round shape, almost echo-free, no fatty hila are seen. Mammography shows focal density in craniocaudal (CC) view, not well defined in mediolateral oblique (MLO) view. CESM shows intense enhancement of that complex lesion, with its size much increased. Numerous enhanced small foci are noted in both breasts. US guided core needle biopsy (CNB) reveals invasive ductal carcinomas and these are confirmed in surgery.
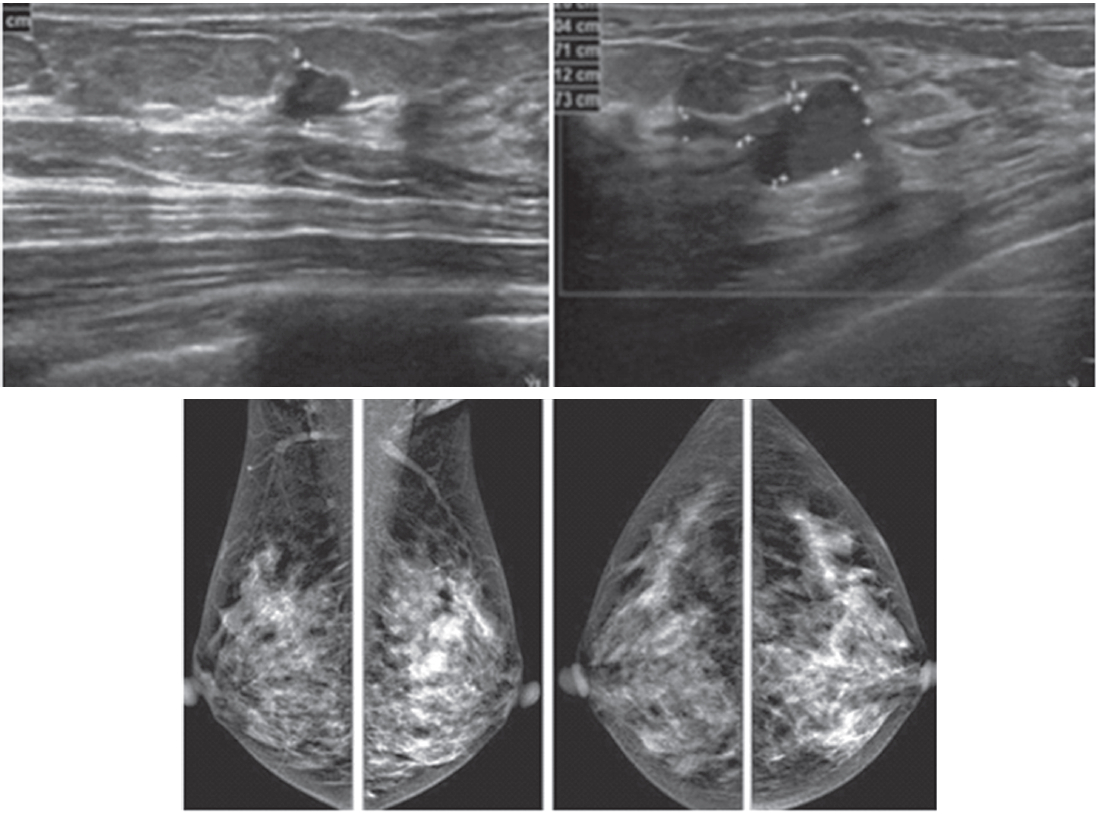
Figure 1B: Female 42-years of age, US shows a relatively large homogeneoushypoechoic solid mass in a cystic lesion with relationship to ducts, measuring 5.2×7.2mm in right upper outer quadrant (UOQ) and 7.3×11.2mm in left UOQ. Mammography shows a density suspected in left MLO at a second look. CESM reveals a moderate degree of uptake of contrast medium in left UOQ, measuring 11.6×11.9mm and an ill-defined focal minimal abnormal uptake in the right breast. CNB reveals fibrocystic changes of both lesions.
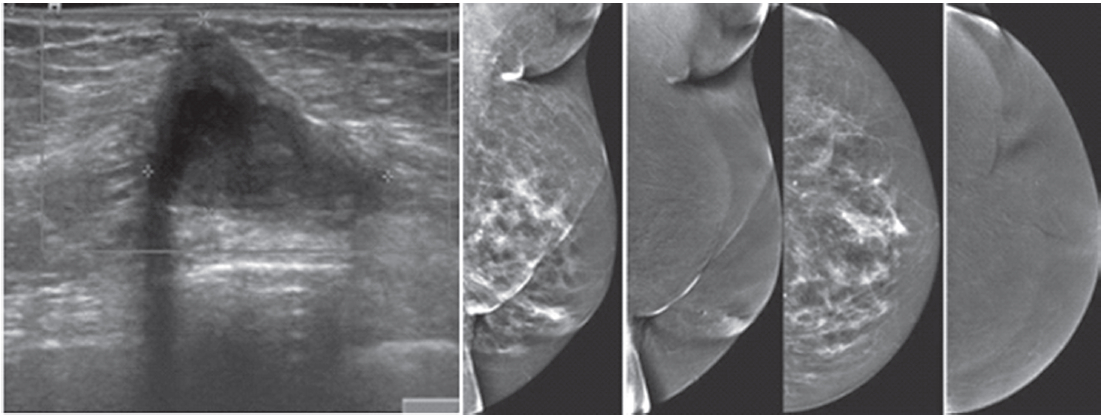
Figure 1C: Female 64-years of age, post op left breast conserving therapy (BCT). Mammography shows architectural distortion at surgical scar in left lower outer quadrant (LOQ). US reveal an enlarging cystic lesion with hypoechoic modularity on its wall and some echoic contents. CESM shows no enhancement of either breast. US guided aspiration yields old hemorrhagic fluid. At surgery, there are no residual malignant cells.
2. Examples of three patients whose US shows heterogeneous hypoechoic solid masses with increased vascularity inside the lesions, the CESM reveals three different findings, indicating different pathologies. The images and final pathological results are presented as follows:
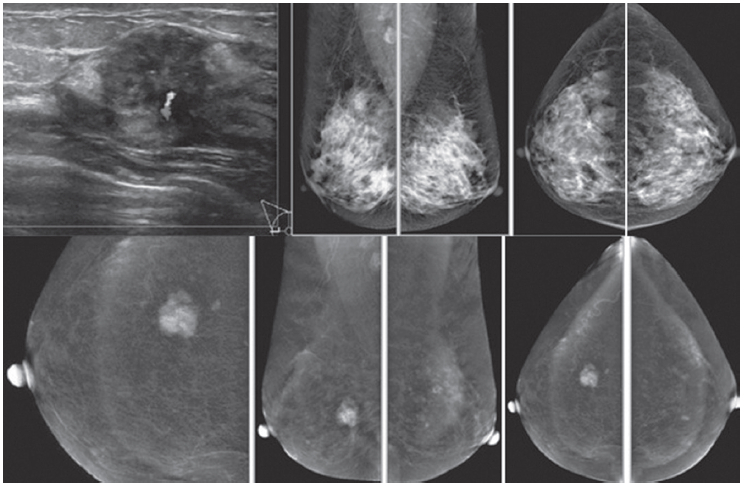
Figure 1D: Female 60-years of age, US shows a well-defined lobulated, minimally heterogeneous hypo-echogenic mass with increased vascularity in right LOQ of 2×1.2×2cm. Mammography shows coarse pleomorphic microcalcifications in the areas of the obscured masses. Axillary nodes are seen in the right MLO. CESM reveals an intensely heterogeneous and enhanced microlobulated mass in right LOQ, measuring 18×22mm, with multiple dark spots of non-enhanced microcalcifications inside the lesion. Multiple foci of mild and moderate enhancement are seen. Soft enhanced right axillary nodes are noted. CNB and surgical pathology reveal invasive ductal carcinoma.
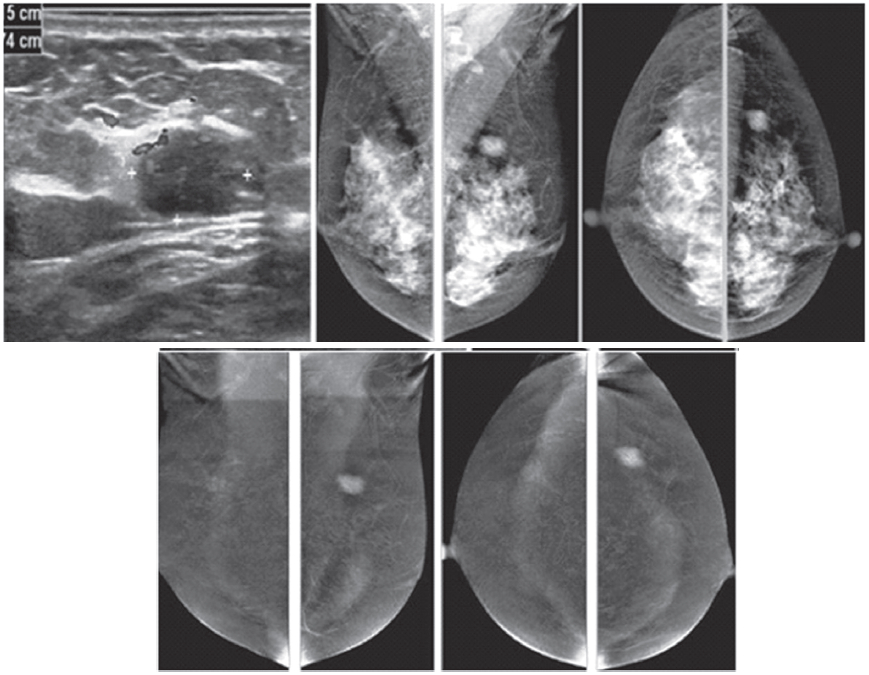
Figure 1E: Female 46-years of age, US shows a well-defined lobulated, minimally heterogeneous hypo-echogenic mass with increased vascularity in the left UOQ. Mammography shows a lobulated isodensity mass. CESM reveals a markedly homogenous enhanced lobulated mass. The outline is smooth and no spiculation is noted. CNB reveals a fibroadenoma.
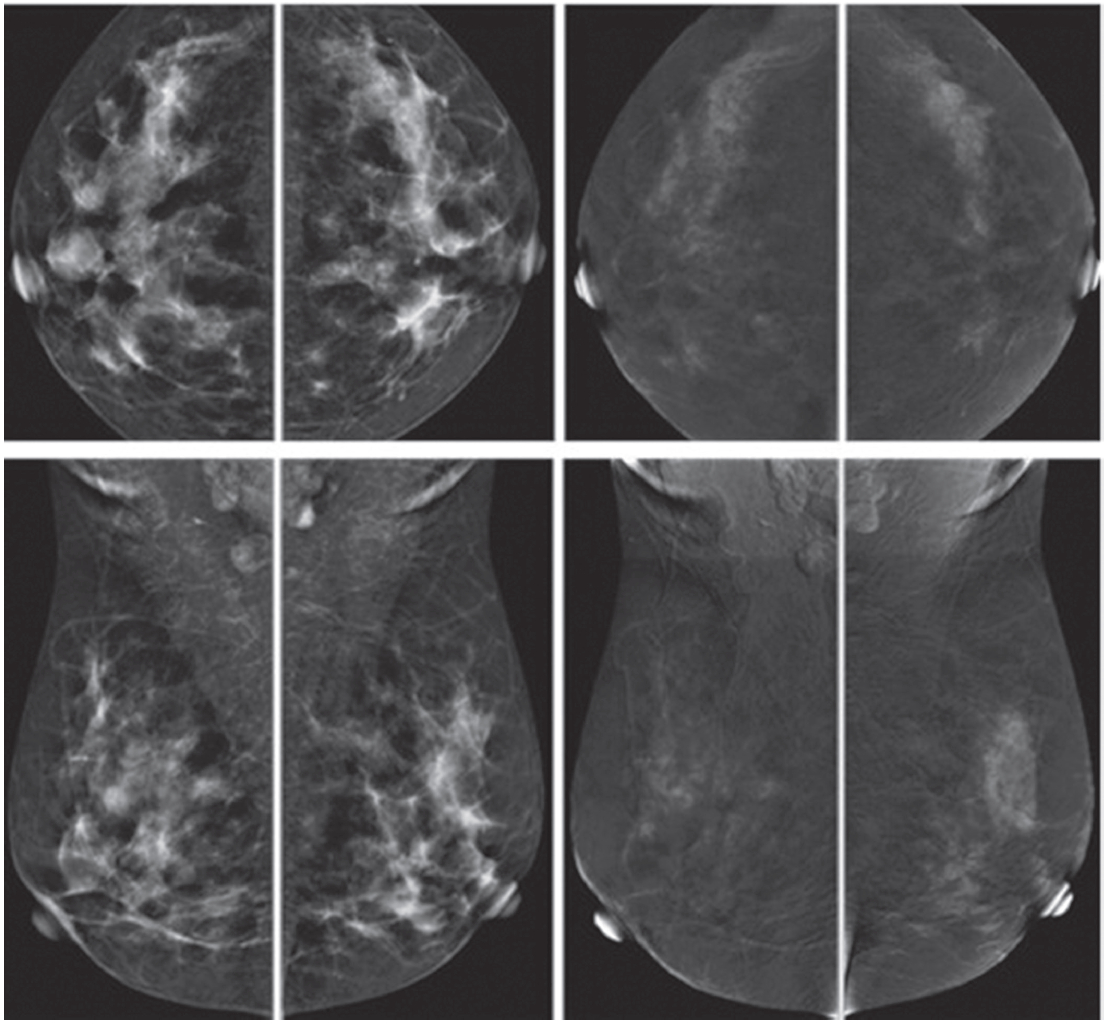
Figure 1F: Female 42-years of age, US shows a well-defined round to oval shaped, minimally heterogeneous hypo-echogenic mass with increased vascularity in the right subareolar area. Mammography shows a round isodense mass in the right SA in CC, partially obscured in MLO. CESM reveals no enhancement of this subareolar lesion a benign finding. CNB reveals fibrocystic changes.
3. Examples of 2 patients whose US show slow hypoechoic lesions, increased depth to width ratio, acoustic shadowing and extensive spiculation. The CESM reveals two different findings, indicating different pathologies. The images and final pathological results are presented as follows:
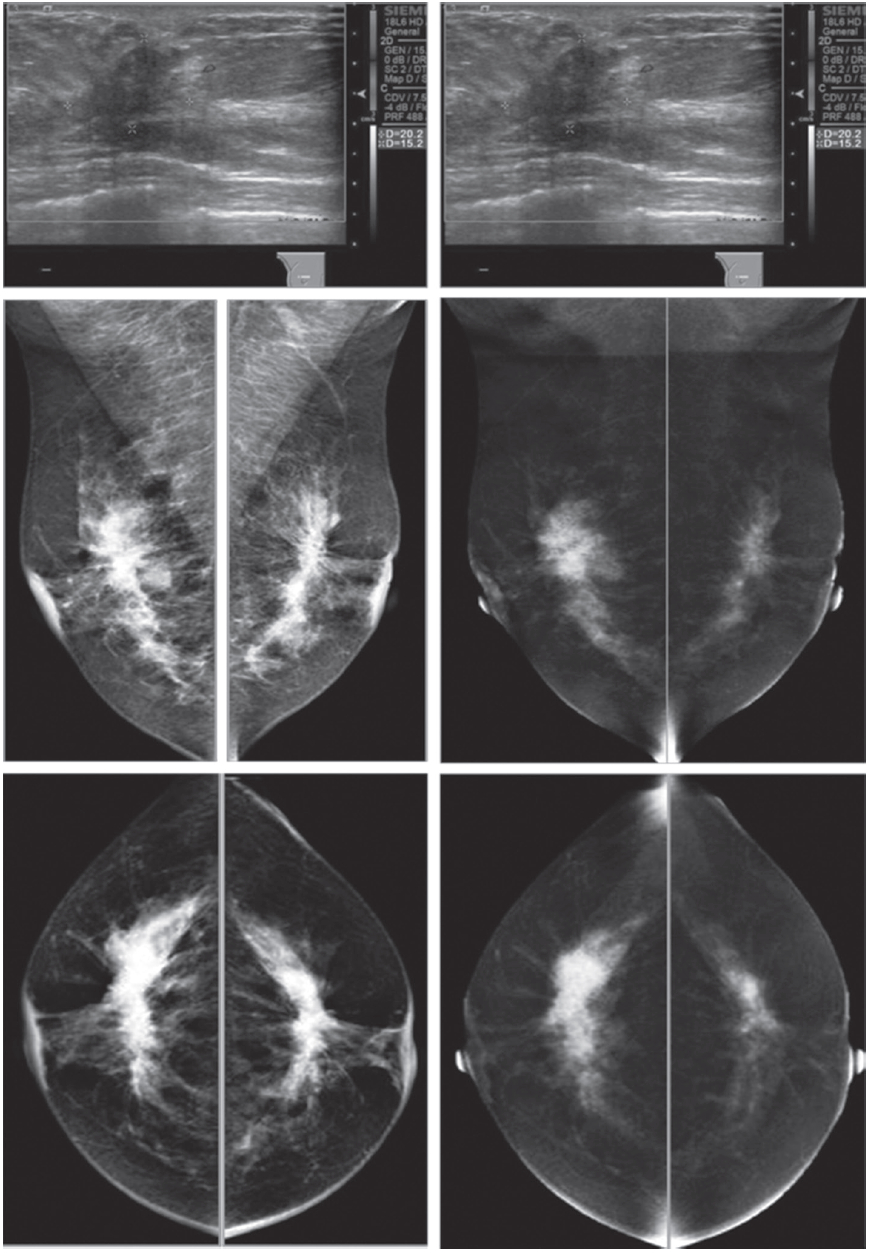
Figure 1G: Female 52-years of age, US of both SA areas show the same findings of a very low hypoechoic lesion with its depth more than width. Acoustic shadowing and extensive spiculation are seen. This may be seen in malignancy and benign lesions such as radial scar or sclerosingadenosis. However the former lesion is enhanced, while the 2 latter lesions show no significant enhancement. Mammography shows extensive breast asymmetry, irregular shaped lesion with extensive spiculation. CESM reveals very high uptake of contrast medium in almost the whole fibroglandular tissue in both breasts, with long spiculations to nipple and skin on a deep to posterior aspects, compatible with extensive involvement of malignancy. CNB reveals bilateral extensively invasive ductal carcinoma, grade II.
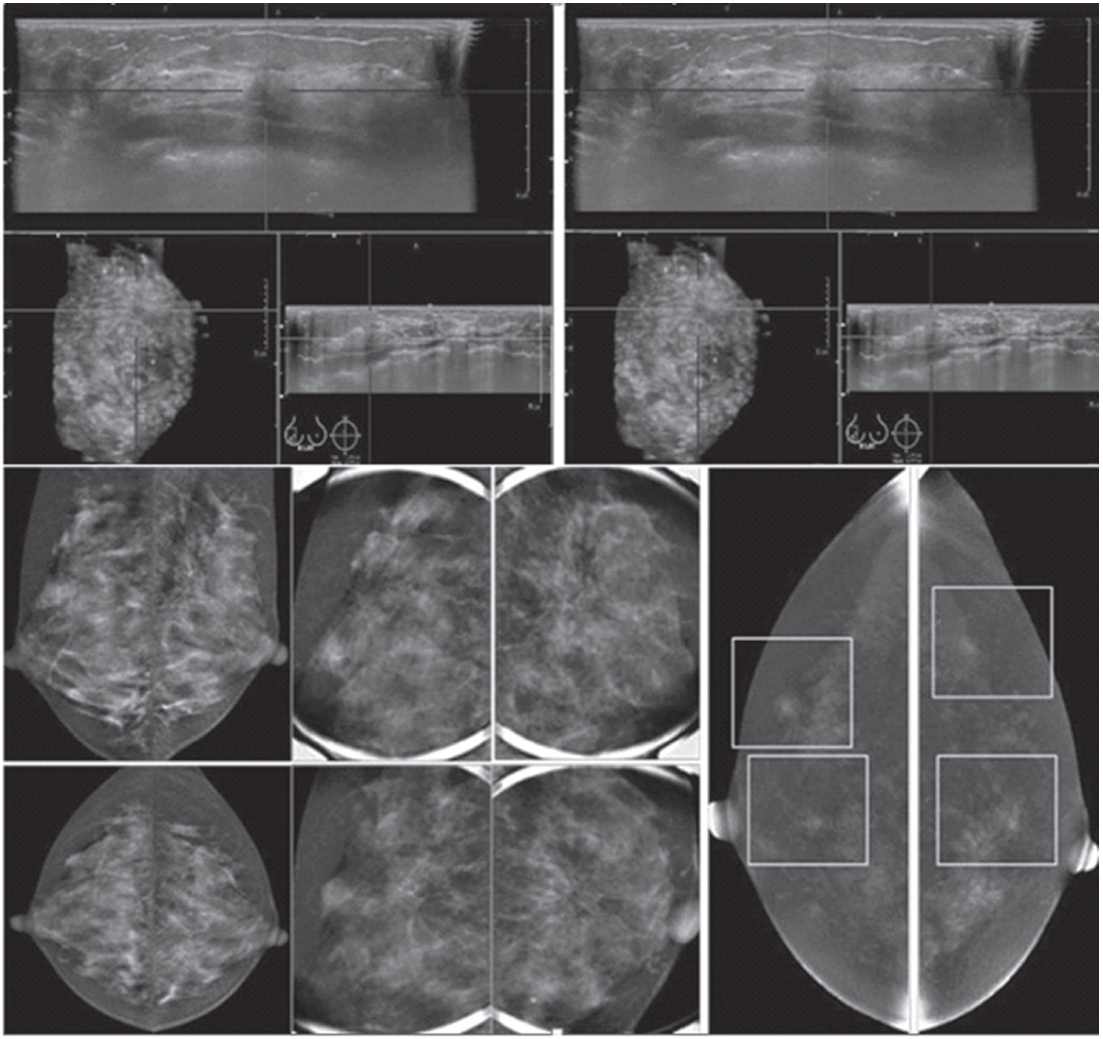
Figure 1H: Female 34-years of age, the automated breast volume scanner (ABVS) shows 2 poorly defined focal hypoechoic areas in left UIQ and right upper, associated with disruption of parenchyma and a lobulated hypoechoic mass in right UOQ: 7×9.9×12.6mm Mammography reveals architectural distortion in both areas, seen with no mass in a six category classification (SCC). CESM reveals multiple focal enhanced nodules of varying degrees, scattered in both breasts. Pathological study reveals a fibroadenoma with sclerosingadenosis.
In the detection and evaluation of the extent of breast cancer, apart from an evaluation of the extent of the cancer as mentioned earlier, DCE-MRI can identify occult contra- lateral cancer in 3-5% of cases12,13 MRI has been considered limited in the evaluation of DCIS. However, more recent studies found MRI superior to mammography in detecting unsuspected DCIS.14,15 The mammographic sensitivity for detecting invasive lobular carcinoma is around 34% to 81%, which is inversely related to mammographic density.
Conversely, the reported sensitivity of MRI for invasive lobular carcinoma is 93% to 96%.4,16 Dillon et al. reported that positive surgical margins occurred in approximately 50% of patients with invasive lobular carcinoma who did not undergo preoperative MRI and 25% in invasive ductal carcinoma.17 MRI evaluates tumor size more accurately than mammography and ultrasound.4,18 Again, considering the same basic principles as CE-MRI, the examples of CESM in the detection and evaluation of the extent of breast cancer are as follows:
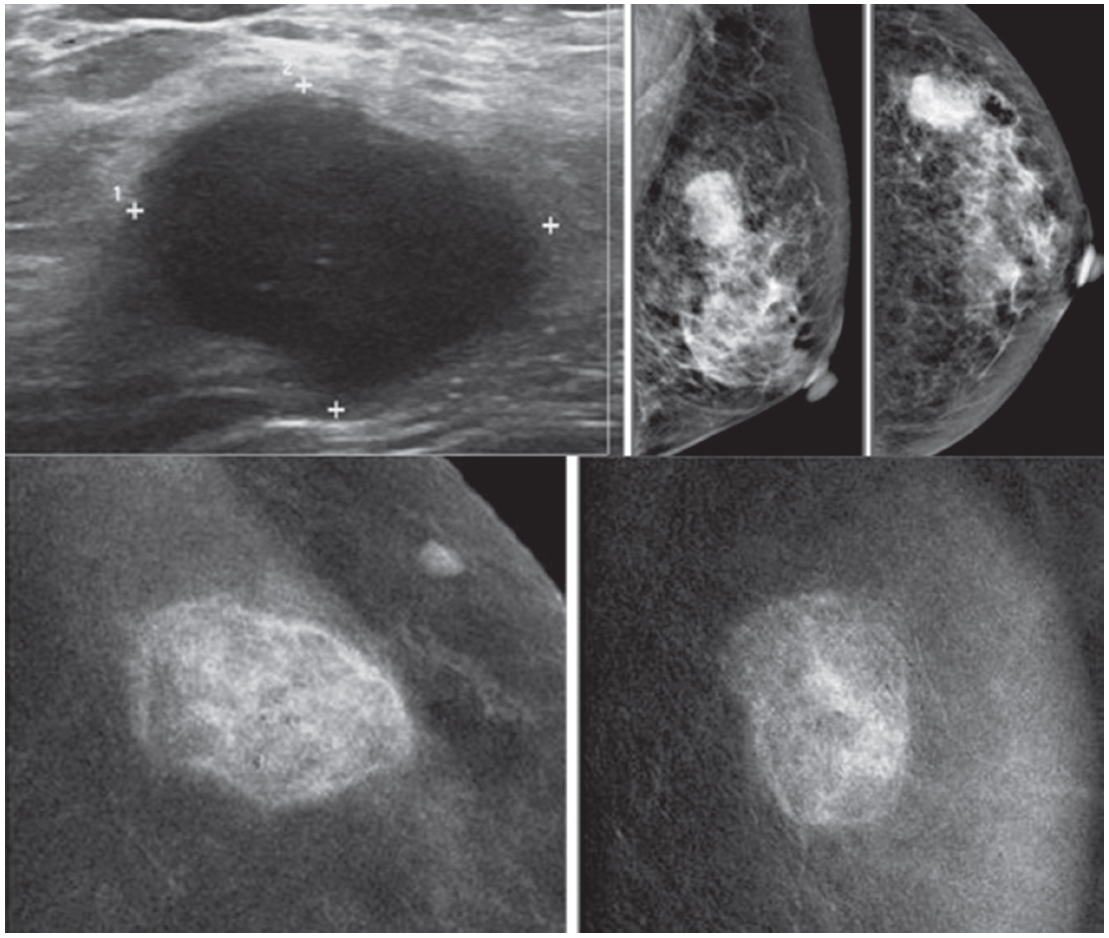
Figure 2A: Female 60-years of age, a mass is palpable in left UOQ Mammography shows a lobular shaped hyperdense mass and US shows an echo-free lobular mass with surrounding tissue reaction, combined type of posterior enhancement. CESM reveals an intensely heterogeneous enhancement of a microlobulate mass of 33×24×22mm in right UOQ, seen with multiple dark spots of non-enhanced microcalcifications inside the lesion, highly suggestive of malignancy. Another small markedly enhanced nodule is seen, which is not noticed initially by mammography and US. There is no abnormal enhancement of the entire right breast and axillary l.n. CESM defines the nature and existing of two cancers in the same breast.
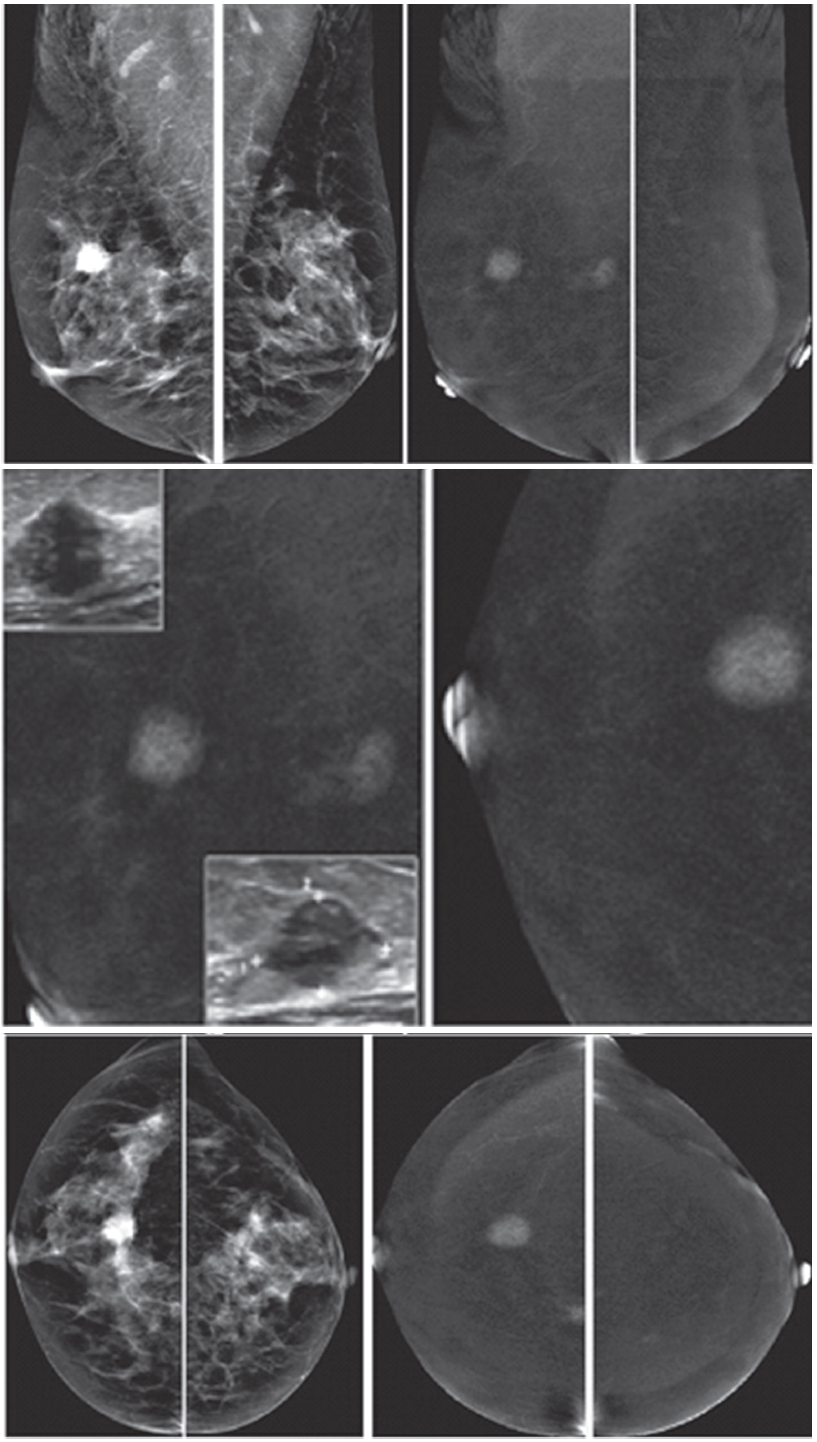
Figure 2B: Female 60-years of age, S/P left CBS, a round mass with microlobulation and spiculation is seen in right upper. Multiple equivocal nodes are seen in both axillae. CESM reveals aheterogeneous uptake of contrast medium in right upper of 16×17mm. An additional lesion is seen in right inner: 9×11mm. No abnormal uptake in left breast and axillary l.n. Second look US reveals two heterogeneous hypoechoic masses with microlobulated outlines. US guided CNB reveals invasive ductal carcinoma of both lesions. CESM detects the second primary multifocal cancers in contralateral breast.
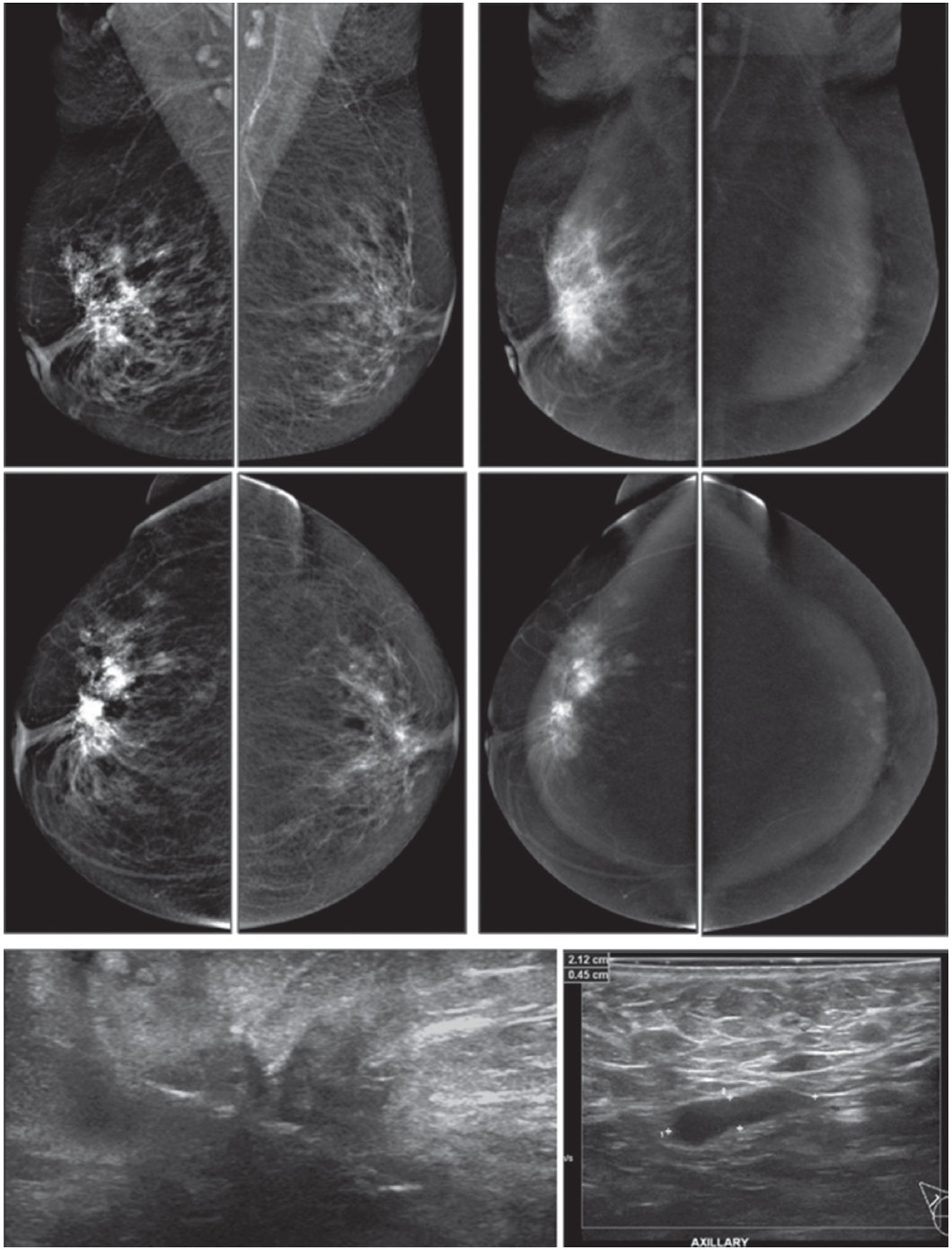
Figure 2C: Female 55-years of age, mammography shows a large irregular shaped focal asymmetry with spiculation in right breast. Ultrasound shows a large spiculated mass. The number of small axillary nodes has increased. CESM reveals an intensely heterogeneous enhancement of a large irregular-shaped mass with multiple dark spots of non-enhanced microcalcifications inside the lesion. Spiculations are seen around the lesion; extending anteriorly to skin and nipple and posteriorly to deep structures. Soft enhanced foci in both breasts and left axillary l.n. are noted, highly suggestive of malignancy. US guided CNB reveals invasive ductal carcinoma. CESM defines the nature, local extension and existence of this cancer, possible bilaterally.
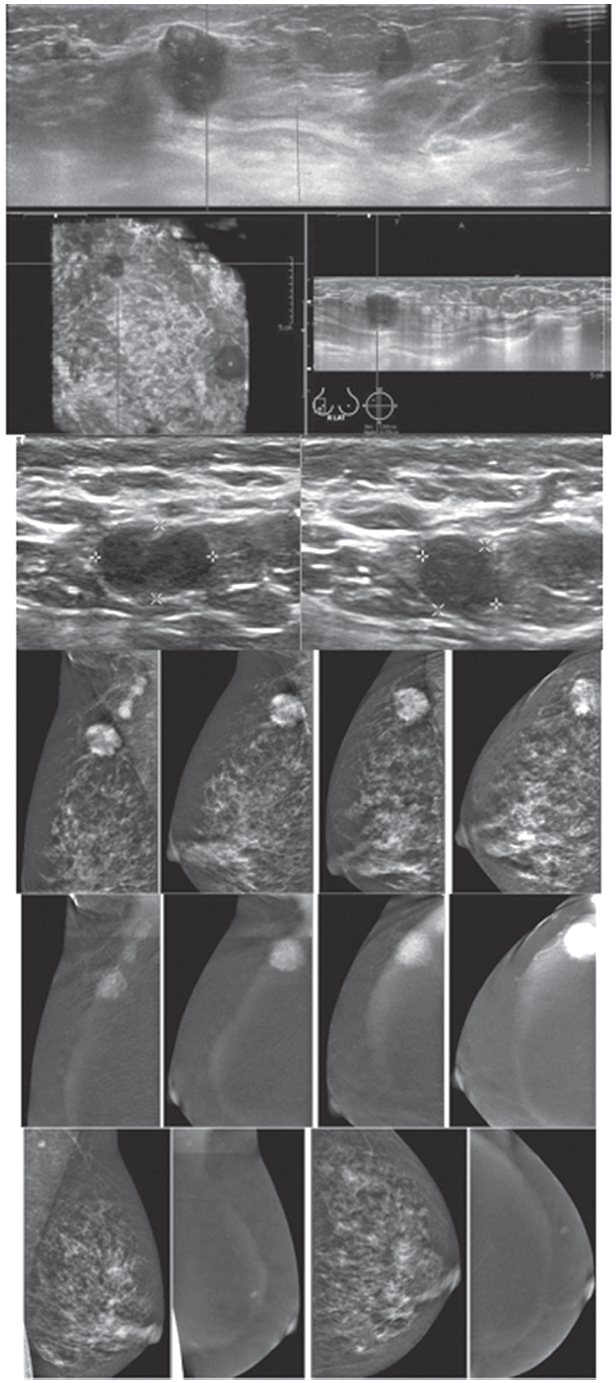
Figure 2D: Female 50-years of age, mammography and ABVS reveal a malignant appearing mass under 2 cm. in right UOQ, however, multiple enlarged adenopathy is noted. CESM shows the malignant nature of the mass as well as enhancement of multiple axillary adenopathy. Apart from that, another enhanced focus is seen in left breast. A tiny left axillary l.n. is minimally enhanced. Surgical pathology confirms invasive ductal carcinoma grade II with l.n. metastasis in 12 out of 18 removed nodes. CESM defines the nature of this small cancer, but with extensive adenopathy and possible additional small lesion in contralateral breast.
When screening patients in high-risk groups, Breast MRI can detect small node-negative cancers in women at high risk for breast cancer and it is a useful screening tool when used as an adjunct to mammography in high-risk women. However, due to its limited specificity and high cost, MRI is not appropriate for screening the general population.19,20 The American Cancer Society (ACS) has recommended annual screening breast MRI for very high-risk women, which includes:
- Women with breast cancer BRCA1 and BRCA2 gene mutations and their untested first-degree relatives
- Patients with prior chest radiation between the ages of 10 and 30
- Those with certain syndromes associated with propensity for breast cancer and other genetic muta-tions, including p53 and Cowden
- Patients with a lifetime risk for breast cancer of >20% to 25% as determined by risk models
For patients with these risk factors there is sufficient evidence to recommend annual CE-MRI in addition to annual mammography for screening for breast cancer. Insufficient evidence was found to recommend for, or against, screening MRI for women at intermediate risk, which included:
- Those with a lifetime risk for breast cancer of 15% to 20% definedby risk models
- Prior diagnosis of atypical ductal hyperplasia (ADH) or lobular carcinoma in situ
- Patients with dense breasts on mammography
- Patients with a personal history of breast cancer
The decision for screening these patients with CE- MRI should be made on a case-by-case basis. Screening with breast MRI is not recommended in women with <15% lifetime risk of breast cancer.
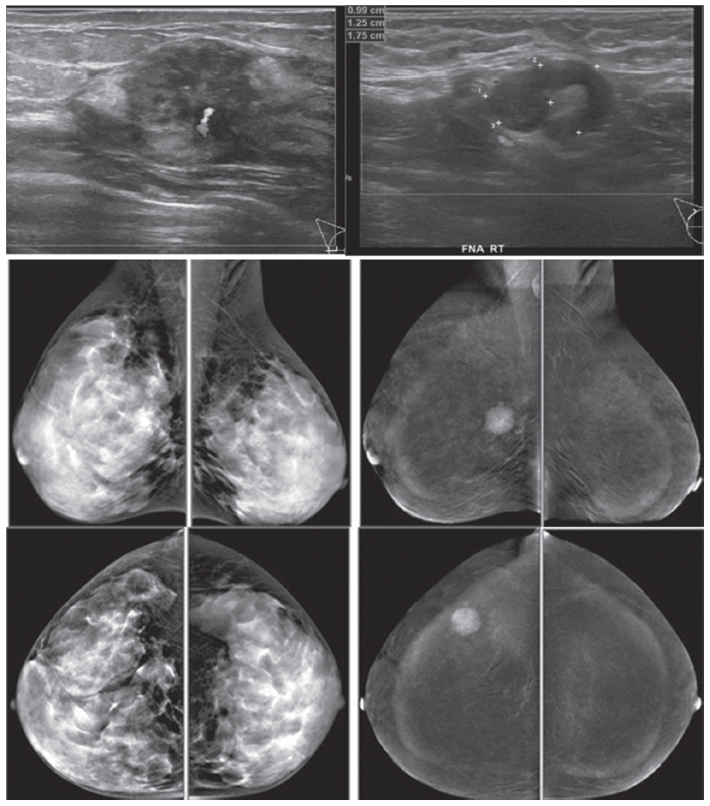
Figure 3: Female 45-years of age, screening in patient with family history of breast cancer. Mammography shows extremely dense breast, no lesion was detected initially. US reveals a round heterogeneously hypoechoic mass in right outer with abnormal vessel inside the lesion. A round axillary l.n. is seen with increased cortical thickness and focal bulging of the cortex, compatible with micrometastasis. CESM reveals an intensely, heterogenous enhanced round mass of 16x19x20.5mm., with partially seen enhanced axillary l.n. US guided CNB reveals invasive ductal carcinoma. CESM demonstrates a cancer with axillary adenopathy in a high risk patient, while the clinical examination and mammography is negative.
When examining the breasts of patients with histologically proved metastatic breast cancer with unknown primary origin, patients presenting with metastatic axillary adenocarcinoma with no evidence of breast cancer on physical exam or mammography represent less than 1% of all breast carcinoma cases. The identification of occult primary breast cancer by MRI is 62% to 86% of patients.21,22 When the primary tumor found by MRI is less than 2 cm, the patient has a choice of breast conservation surgery as a treatment option with targeted hormonal and chemo-therapeutic treatments. However, identifying the primary breast tumor will not affect the prognosis when axillary node involvement is already present.
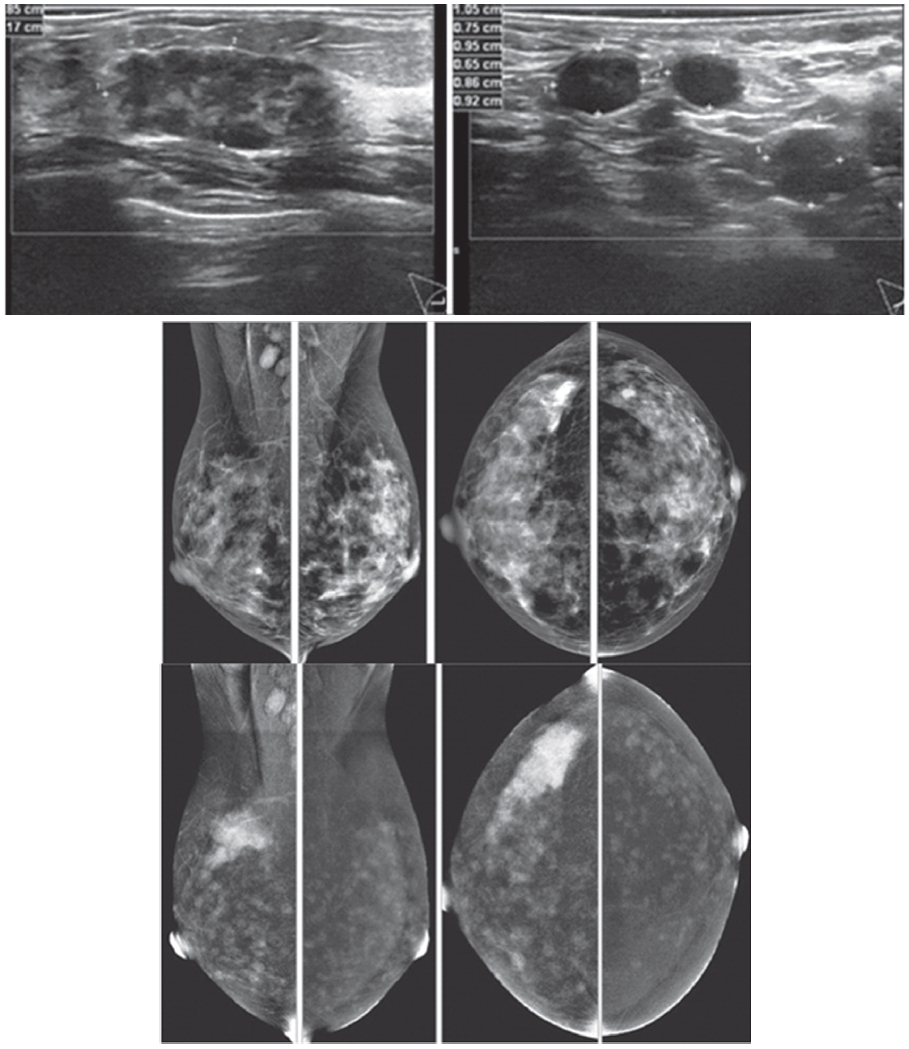
Figure 4: Female 48-years of age presents with palpable right axillary l.n. US confirms numerous axillary adenopathy and a welldefined mixed echoic mass in right UOQ. Mammography shows a focal density in right CC, obscured in the first look at right MLO. CESM reveals an intensely heterogenous enhancement of a large irregular shaped mass, seen with some dark spots of non-enhanced microcalcifications inside the lesion. Enormous moderately enhanced foci are seen in both breasts. The study was performed near the menstruation, thus repeat study should be performed to differentiate extensive bilateral cancer or physiological enhanced foci due to hormone effect. The partially seen enhanced right axillary nodes are extensive. CESM detects and confirms the unconvincing mammography abnormality, axillary adenopathy and possible extensive bilateral lesions or hormonal effect.
Residual disease post-lumpectomy, followed by radiation, is an acceptable choice in the treatment of stage I and II breast cancer and has been shown to provide the same survival rates as radical and modified radical mastectomies.23 The rate of positive margins is around 40% of lumpectomies with increased chance of local recurrence. Breast MRI may reveal the presence of multifocal and multicentric disease as well as detect residual disease at the lumpectomy site, which is necessary information for judging whether re-excision or mastectomy is required. If microscopic residual disease at the surgical margins is present, surgical excision is still required, even though the MRI is negative. The sensitivity for detecting residual disease is around 61% to 86%.24,25 Before 28 days after the operation, the granulation tissue may mimic residual tumour, causing false-positive study. Frei et al. stated that the false-positive results are decreased when MRI was performed between 35 to 42 days following surgery.26
In response to neoadjuvant chemotherapy, a breast MRI is helpful in demonstrating the tumor size, identifying residual tumor following the completion of neoadjuvant therapy. The accurate correlation with pathologic specimens is 71% to 90% for CE-MRI, 19% to 60% for clinical exam, 35% to 75% for ultrasound and 26% to 70% for mammography.27-30 The MRI, however, tends to overestimate the size of residual disease because of the antiangiogenic effects of certain chemotherapeutic agents on the tumor, therefore the ability of DCE-MRI to evaluate lesion enhancement can be significantly lower. As was mentioned prior in the positive tumour margin, even though no residual disease is seen by MRI, surgical resection is still required due to the potential under-estimation of residual disease. So it is necessary to place a marker at the tumour site prior to treatment.
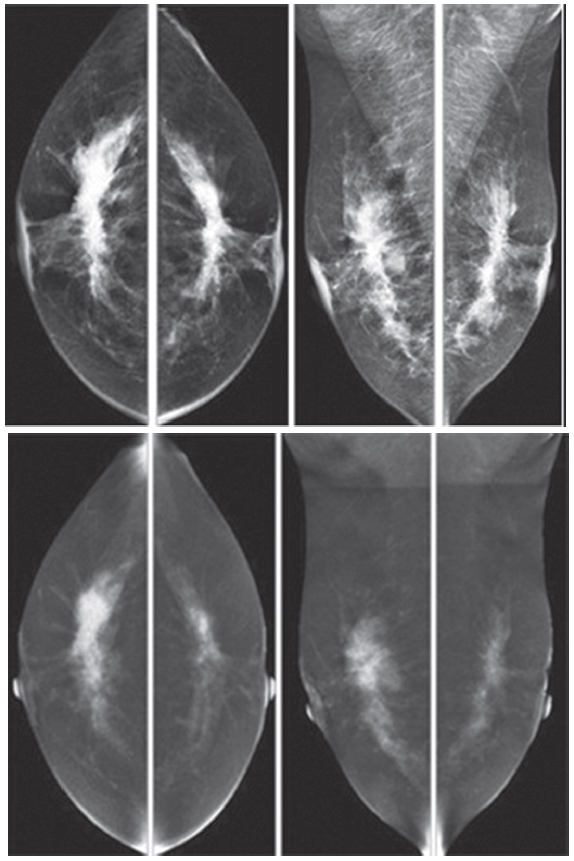
Figure 5: Female 52-years of age, CESM reveals very high uptake of contrast medium in almost the whole fibroglandular tissue in both breasts, with long spiculation to nipple, skin and deep to posterior aspects. CNB reveals bilateral extensive invasive ductal carcinoma; grade II, not suitable for surgery. Chemotherapy was given and CESM will be performed to evaluate the results of the treatment.
For the detection of cancer recurrence after treatment, including postoperative tissue reconstruction, in patients who have undergone mastectomy with a transverse rectus abdominismyocutaneous flap (TRAM), latissimusdorsi flap, or gluteal flap, the follow-up by mammography gives only limited information in detecting recurrence,31 but it can be used as part of routine surveillance in patients with a history of breast cancer. CE-MRI is helpful in identifying local recurrent disease, especially at the chest wall32 and differentiating the coincidental finding of benign lesion from cancer recurrence.
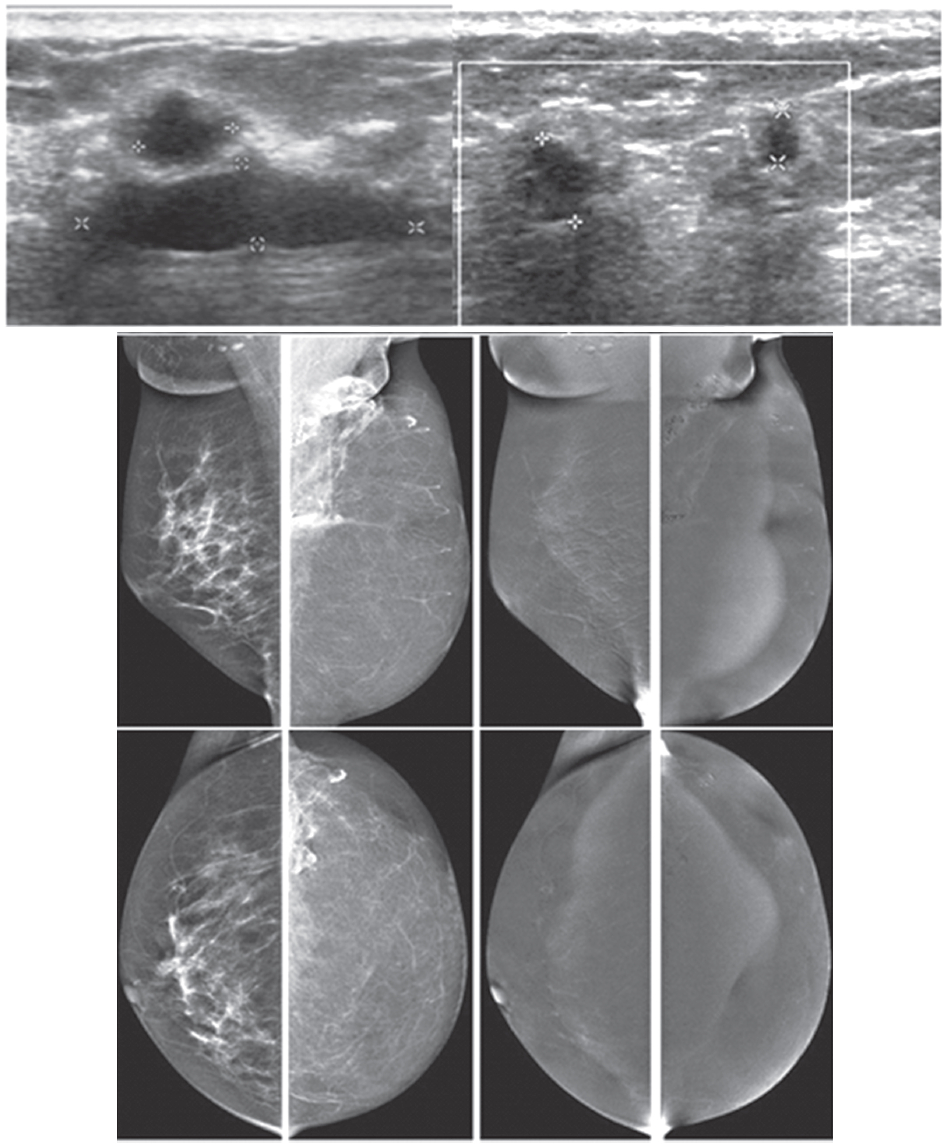
Figure 6A: Female 51-years of age, S/P left MRM with TRAM flap for left breast cancer. Mammography shows dystrophic calcified areas in far left UOQ US shows 9 lobulated echo-free lesions with wall thickening. There is no typical posterior enhancement that is usually seen in cystic lesions. CESM reveals no significant enhancement of both breasts, including at the dystrophic calcified area, compatible with oil cysts post TRAM flap, no evidence of recurrence. CESM post TRAM flap operation excludes local recurrent in multiple oil cysts with dystrophic calcifications.
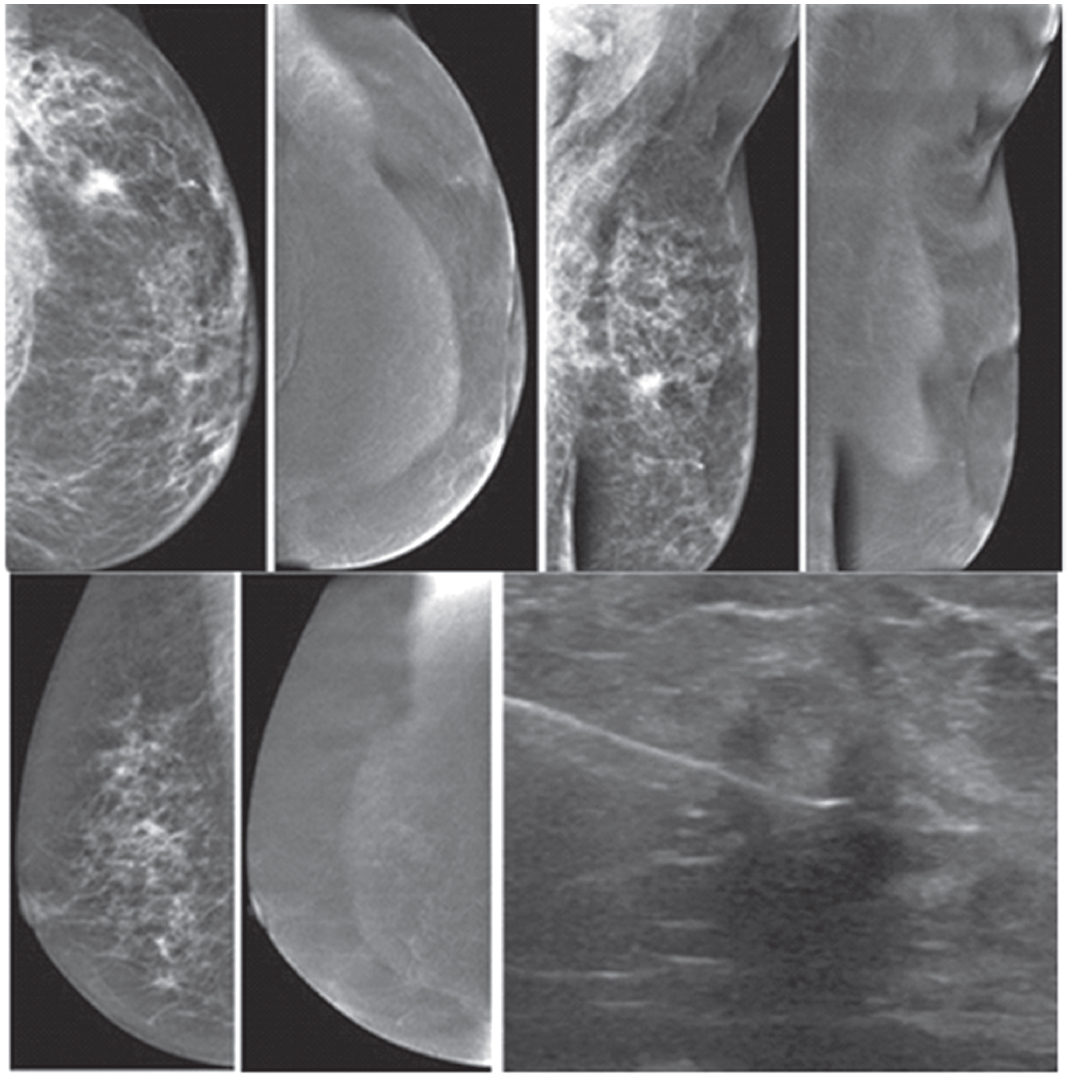
Figure 6B: Female 60-years of age, S/P left MRM with tram flap. Mammography shows a small spiculated mass in PO area, noted with other PO changes. CESM: No abnormal enhancement in both breasts, over all non- malignant lesions. U/S guided CNB of an irregular-shaped markedly hypoechoic mass reveals no residual cancer. CESM post TRAM flap operation excludes local recurrent in the PO spiculated mass.
With the differentiation between scar tissue and local recurrent cancer after breast-conserving therapy, post- operative changes may mimic breast cancer recurrence at the lumpectomy site by conventional imaging. MRI is useful in differentiating recurrent disease from post- operative scarring; however it may enhance MRI for 1-2 years following surgery. A negative MRI may be helpful in excluding recurrent disease. This may be more difficult when the postoperative scar is still enhancing. In general, a scar tends to present as a thin rim or cloud of enhancement around the cavity, whereas a recurrent tumor tends to be more clumpy or mass-like.
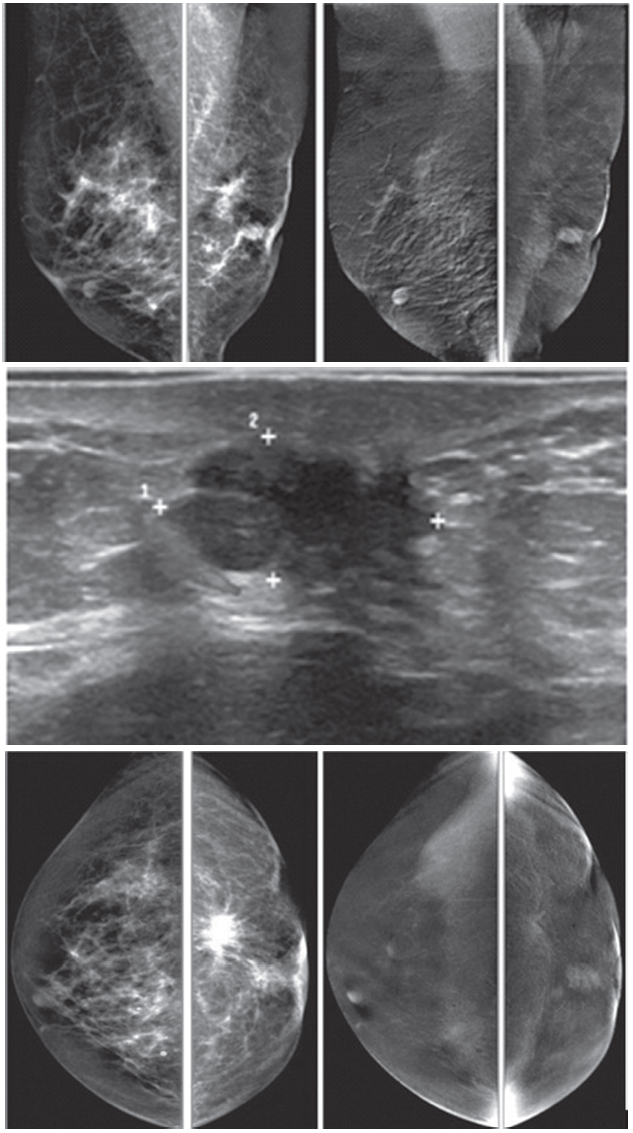
Figure 7A: Female 54-years of age, S/P lumpectomy in the left upper quadrant noted with palpable abnormality in PO area, not at the left subaredar SA. US shows an irregular mass-like lesion with spiculation in left central at post operation scar. A well-defined heterogeneous hypoechoic lobulated mass is noted in left SA: 8.1 x 16.2 mm CESM reveals the irregular mass-like lesion with spiculation in left central is not enhanced while the non-palpable nodule is densely enhanced in heterogeneous pattern. (The skin mole in right LOQ is seen with enhancement.) At surgery, the post-operative scar shows no malignancy, while the non-palpable SA mass is a recurrent invasive ductal carcinoma. CESM confirms no local recurrence, but another recurrent cancer is noted, slightly away from the PO scar.
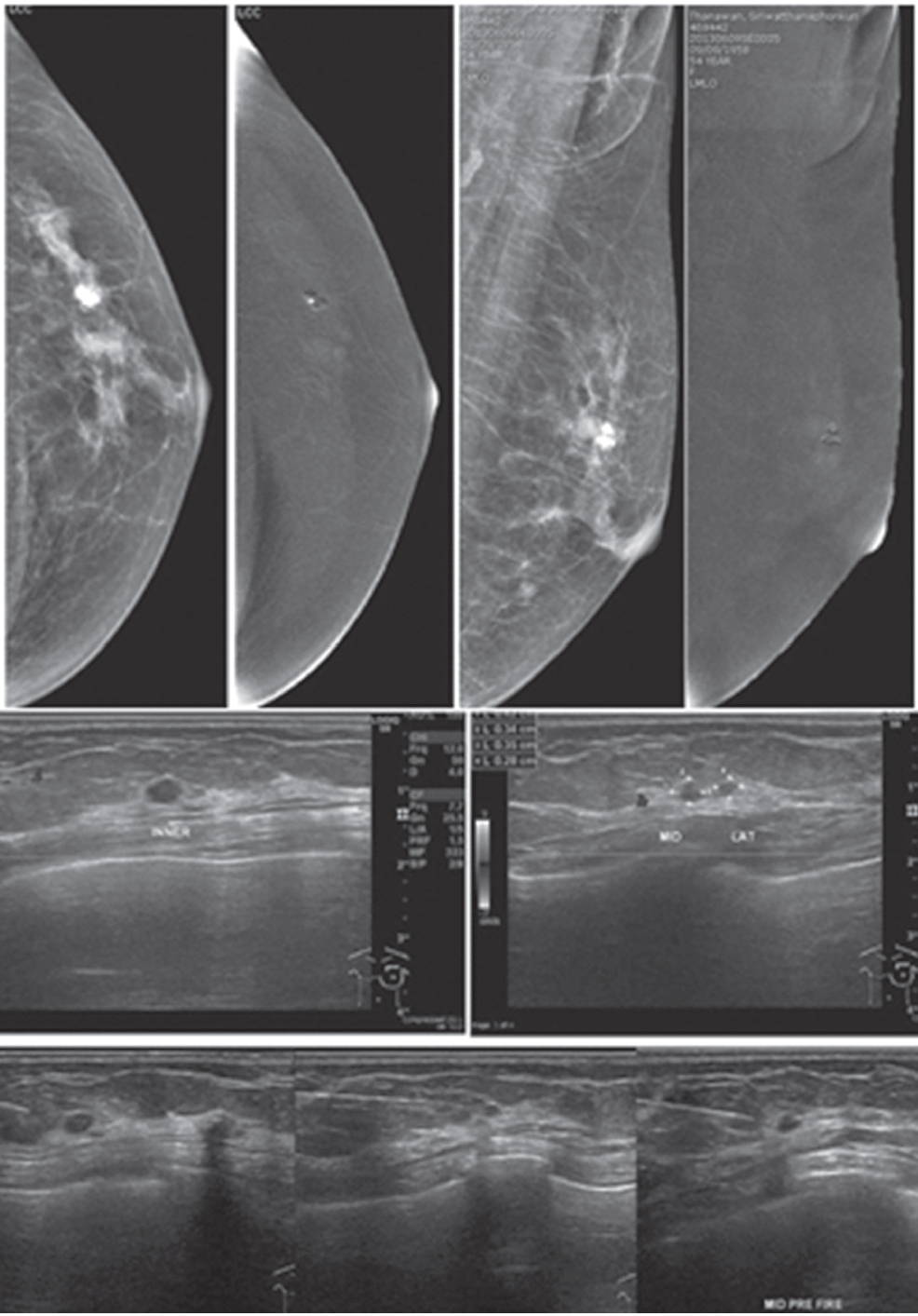
Figure 7B: Female 54-years of age, S/P left MRM for IDCA. Mammography reveals popcorn calcifications and a cluster of round microcalcifications in left UOQ. Breast US shows 3 microlobulated heterogeneous hypoechoic masses in left upper inner: 4.3×4.9×5mm, left upper middle: 3.1×4.3mm and left upper lateral: 3.1×4.5mm. CESM reveals very soft enhancement of these 3 tiny nodules. US guided CNB of these 3 lesions reveal IDCA, moderately differentiated in all specimens.
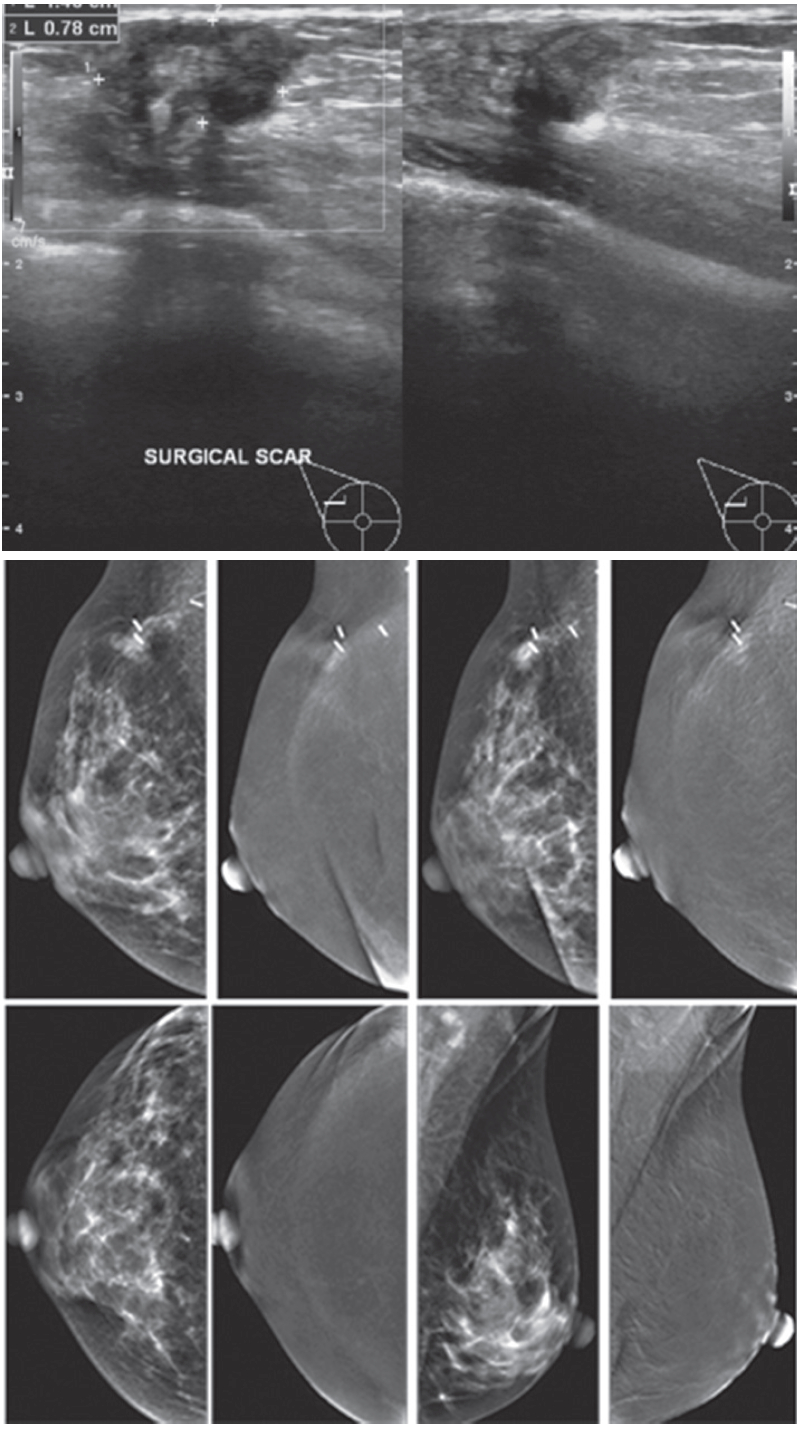
Figure 7C: Female 36-years of age, S/P right CBS for breast cancer came with palpable abnormality at PO area. US shows a heterogeneous hypoechoic mass, lobulated outline, near PO scar, 14.0x7.8 mm. with multiple heterogenous hypoechoic masses in both breasts, and recurrence cannot be excluded. CESM reveals soft enhancement at the PO area with palpable abnormality and surgical clips. There is no significant abnormal enhancement in the rest of both breasts. At surgery, there is no local recurrence. CESM confirms no local recurrence in equivocal mammography and US findings, as well as no evidence of malignancy elsewhere.
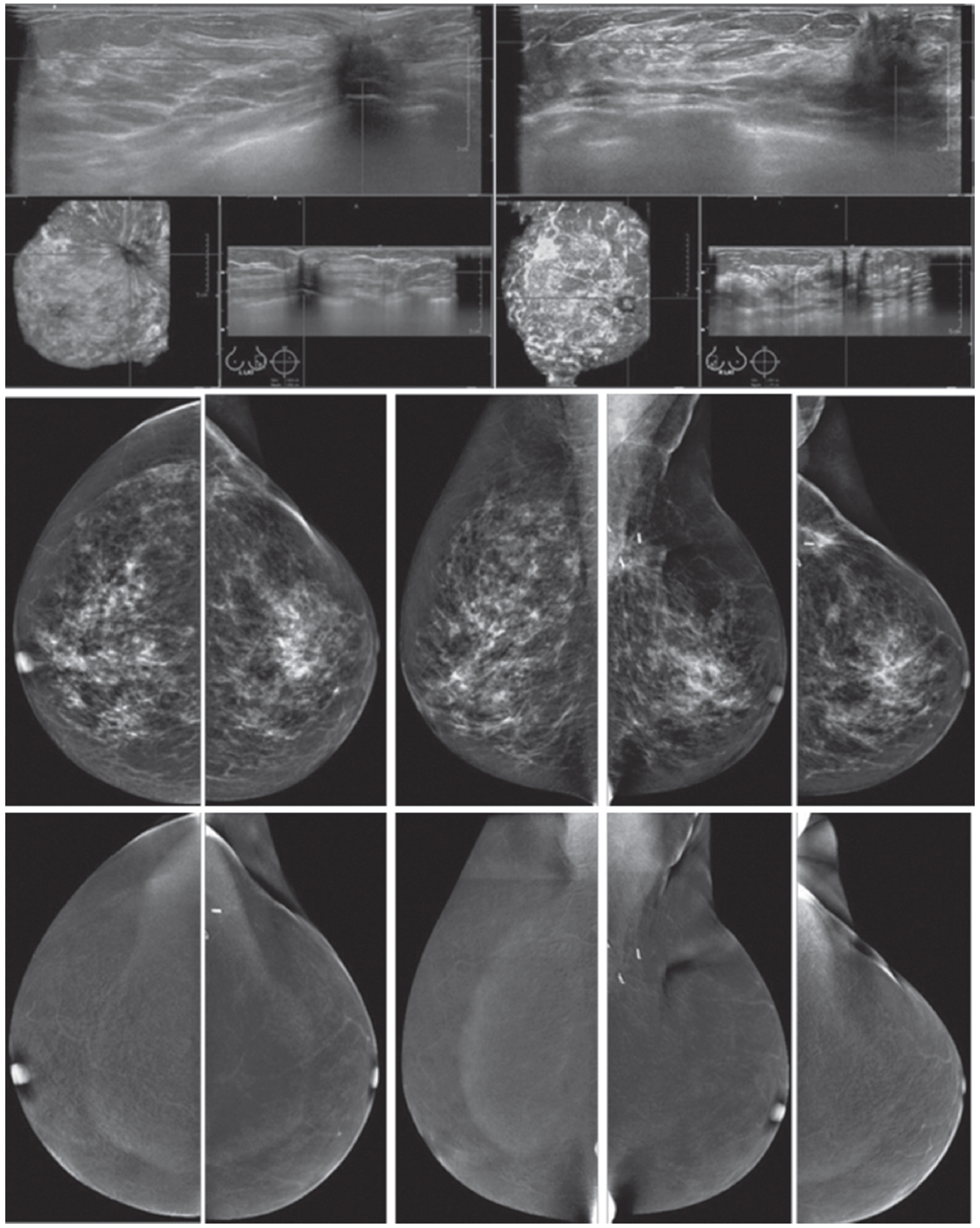
Figure 7D: Female 46-years of age, S/P left UOQ lumpectomy and left sentinel node biopsy for IDCA, grade II US shows an irregular mass--a lesion with spiculation in left central at post operation (PO) scar. Mammography shows architectural distortion at surgical scar with surgical clips in left UOQ.
Benign-looking punctuated microcalcifications are noted in left outer; CESM reveals no enhancement at PO area in left UOQ and elsewhere in the breasts, compatible with no local recurrence or tumour elsewhere in the breasts. CESM confirms no local recurrence at PO scar with a tiny spiculated lesion and no evidence of malignancy elsewhere.
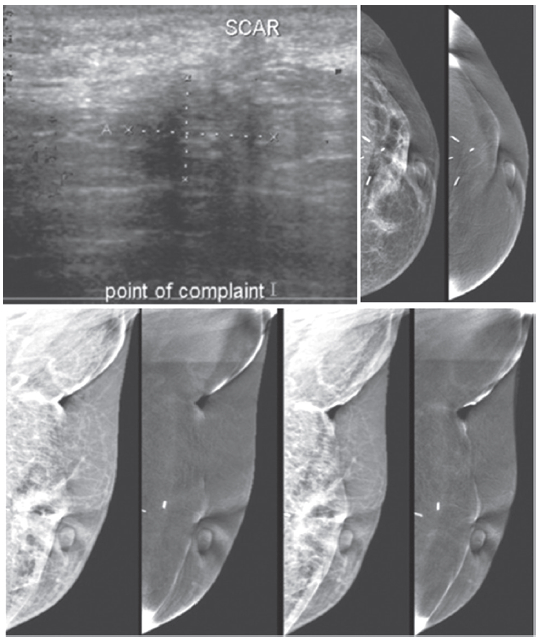
Figure 7E: Female 56-years of age, S/P left BCT for invasive ductal carcinoma, came with palpable abnormality in left axilla. US shows an ill-defined abnormal echoic area, no confined mass in left axillary area, at the palpable abnormality, measures 11×16mm. Mammography shows PO changes, no associated mass.
CESM shows no abnormal uptake of contrast medium, compatible with no malignancy, and CESM confirms no local recurrence at palpable abnormality around the PO scar and no evidence of malignancy elsewhere.
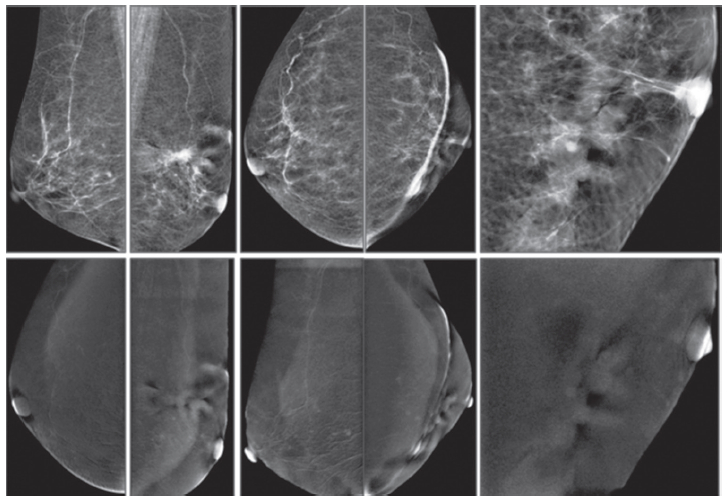
Figure 7F: Female 74-years of age, mammography shows a spiculated mass in PO area with severe deformity, skin thickening and thick strand extends to the nipple. CESM shows no enhancement of the mentioned areas. No local recurrence is detected. However, a few tiny soft enhanced foci are seen in both breasts, and this requires close follow-up. CESM confirms no local recurrence at PO scar and but cannot exclude tiny foci of malignancy, and this requires follow-up.
The results obtained with CESM are immensely valuable, with better contrast resolution than CE-MRI. Extremely fine details of microcalcifications are obtained and very tiny spots of enhancement can be seen along the pathological ducts, not visualized in CE-MRI or breast ultrasound. Our study of CESM examinations clearly shows its benefits over CE-MRI and it should be used when CE-MRI is required. With regards to radiation exposure and possible side effects of the already known iodinated contrast medium, we do not use CESM as a routine initial study, replacing mammography. Our routine breast imaging is still mammography, followed by breast ultrasound. If both prove inconclusive or if there is a possibility to reveal additional cancers in patients planned for breast conservative surgery and CE-MRI is requested, then CESM is recommended as a superior choice than CE-MRI alone. There are benefits for patients in shorter examination time and greater specificity in the identification of tumours and additional cancers that may remain undetected using conventional diagnostic imaging methods.