Esophageal cancer is the 8th most common cancer worldwide with an estimated 456,000 new cases in 2012,and is the sixth most common cause of death from cancer with an estimated 400,000 deaths. Esophageal cancer incidence is more common in men than women with the ratio 2.4:1.1 Esophageal cancer five-year survival rates are among the worst reported in any malignancy. According to data from the National Cancer Institute Surveillance,Epidemiology and End Results (SEER) Program, the five-year survival rates for patients with esophageal and gastric cancer improved only modestly over the last 50 years, from 4% in the years 1950 to 1954, to 18.6% in year 2012.2
The majority of esophageal cancer patients presented with advanced disease and therapeutic options are limited. Squamous cell carcinoma (SCC) and adeno- carcinoma account for 93% of all esophageal carcinoma. Squamous cell carcinoma is the most common histology in Eastern Europe and Asia, and adenocarcinoma is most common in North America and most Western European countries. Squamous cell carcinoma seems to be more sensitive to chemotherapy, chemoradiation, and radiation than adenocarcinoma, but the long term outcome appears to be the same. Tobacco and alcohol abuse are major risk factors for squamous cell carcinoma whereas obesity and high body mass index (BMI) have been established as strong risk factors for adenocarcinoma of the esophagus.3-6
A 61-year-old Caucasian male with past medical history of insulin dependent diabetes, hypertension, and chronic kidney disease stage III who presented with 2 months’ history of progressive dysphagia and 10 kilograms (kgs) weight loss in 2 weeks. Patient underwent esophagogastroduodenoscopy (EGD) with biopsy and was found to have fungating friable mass at 28 cm with obstruction of esophageal lumen, unable to pass the nasal endoscope. Biopsy revealed squamous cell carcinoma (Figure 1). PET/CT revealed multiple metastatic lesions including liver, bone and left adrenal gland (Figure 2).
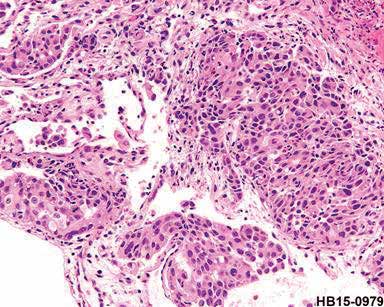
Figure 1: The esophageal mucosa reveals a group of poorly differentiated malignant epithelial cells in the tissue which is likely to be squamous cell, carcinoma, poorly differentiated.
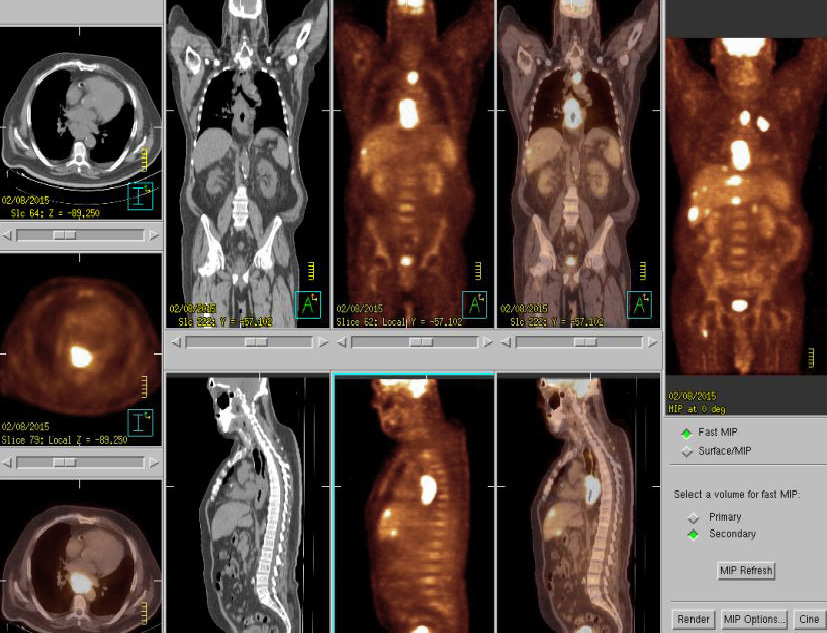
Figure 2: PET/CT at the initial diagnosis suggestive of primary cancer at lower thoracic esophagus with lymph node metastases at paraesophageal nodes, left upper paratracheal and paragastric cardial regions, distant metastases at liver, bone and left adrenal gland.
The patient underwent a total of 9 cycles of chemotherapy with carboplatin and paclitaxel given every 3 weeks. He tolerated chemotherapy quite well except for grade 1-2 peripheral neuropathy and fatigue. PET/CT scan after 6th cycle of chemotherapy revealed very good partial response in all lesions. During chemotherapy cycle 1-8, the patient’s clinical the symptoms were much improved and he was able to gain 6 kgs body weight. However, after the 9th cycle of carboplatin and paclitaxel, PET/CT scan revealed progressive disease at distal esophagus and left 4th rib. Patient also complaint of increase in pain at left 4th rib area. Our multidisciplinary conference suggested concurrent chemoradiation to distal esophagus and left 4th rib lesion. He underwent concurrent chemoradiation with capecitabine. Radiation dose was 4700 cGy in 20 fractions to lower esophagus and 4500 cGy in 20 fractions to left 4th rib. PET/CT scan at 6 weeks after concurrent chemoradiation revealed regressed esophageal lesion but progressed lesion at paraesophageal lymph node, left adrenal gland, left 4th rib (Figure 3).
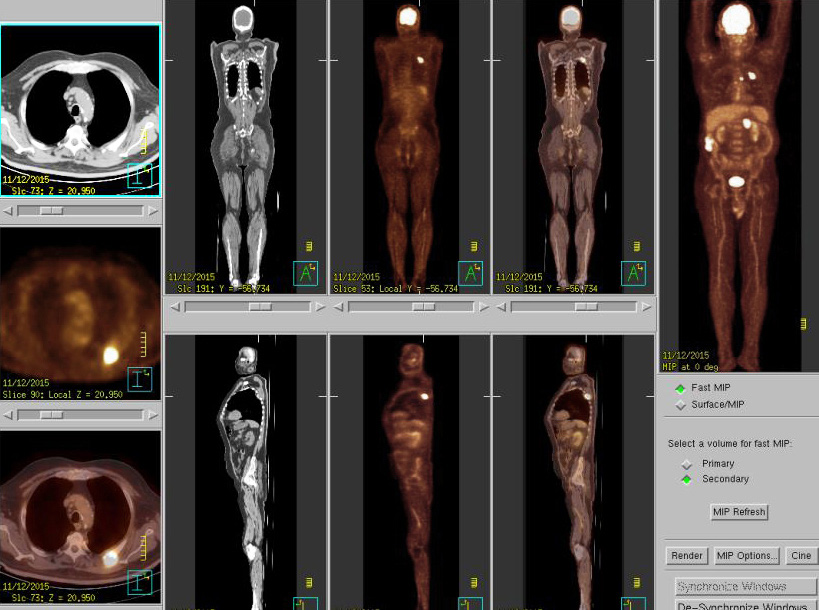 Figure 3: PET/CT after concurrent chemoradiation revealed marked regressed the hypermetabolic circumferential tumor of mid to lower esophagus. Progress metabolic activity with unchanged size of lymph nodes at right paraesophageal nodes and also new found hypermetabolic left adrenal mass. Progressed metabolic metastasis at left 4th rib.
Figure 3: PET/CT after concurrent chemoradiation revealed marked regressed the hypermetabolic circumferential tumor of mid to lower esophagus. Progress metabolic activity with unchanged size of lymph nodes at right paraesophageal nodes and also new found hypermetabolic left adrenal mass. Progressed metabolic metastasis at left 4th rib.
We did a re-biopsy of the lesion at the left 4th rib and sent for MI profile (Molecular Intelligence profile: Caris life science®). Microsatellite instability PCR showed Microsatellite stable. While for MI profile results, we waited the patient underwent third line chemotherapy with docetaxel x 3 cycles. During docetaxel chemotherapy, he developed clinical progression with increase in rib pain, weight loss. PET/CT scan also revealed disease progression at all lesions. MI profile revealed the tumor has a programmed death-ligand 1 (PD-L1) positive 95% which may have potential benefit from anti-programmed cell death protein 1 (PD1) treatment (Figure 4.1-4.3). Therefore, the patient was given pembrolizumab 200 mg IV every 3 weeks. After 2nd treatment, the patient’s clinical symptoms were much improved. PET/CT scan after 6th cycle of pembrolizumab revealed almost complete response at right paraesophageal nodes and disappearance of hypermetabolic activity at left adrenal gland and left 4th rib (Figure 5). Currently the patient has finished 9 cycles of pembrolizumab without significant adverse effect and he still continued to receive treatment every 3 weeks.
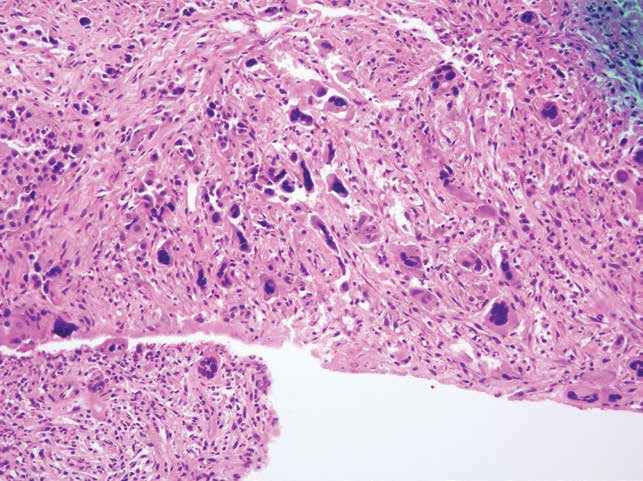
Figure 4.1: Shows H&E.
Figure 4.2: Shows PD-1 immunohistochemistry.
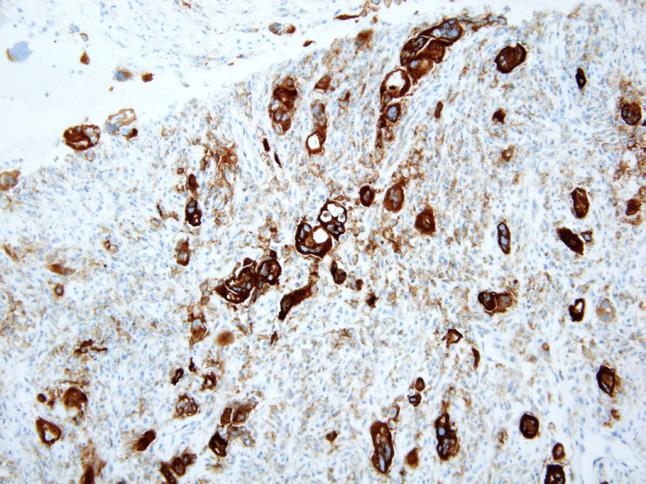
Figure 4.3: Shows PD-L1 immunohistochemistry.
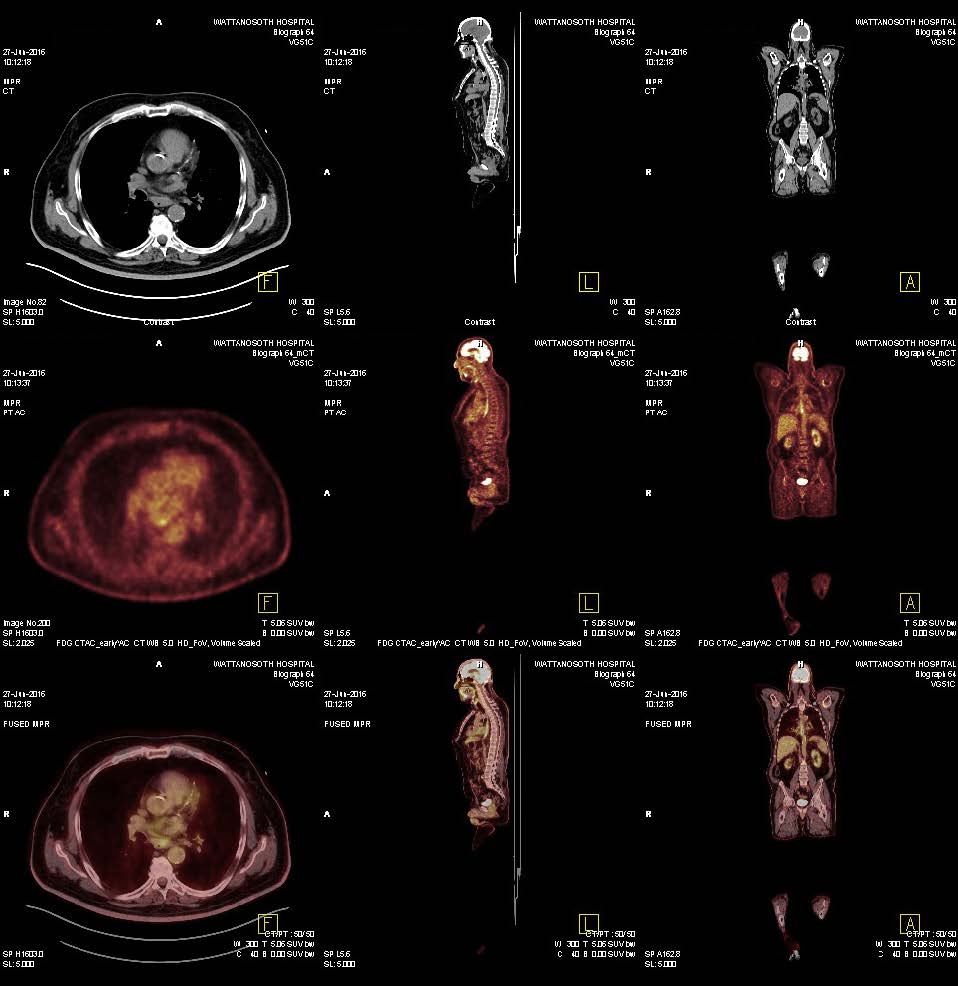
Figure 5: PET/CT after 9 cycles of pembrolizumab (27 weeks of treatment) showed markedly regressed metabolic activity of the pre-existing node metastasis at right paraesophageal areas. Disappearance of hypermetabolic activity at left adrenal gland and left rib are observed.
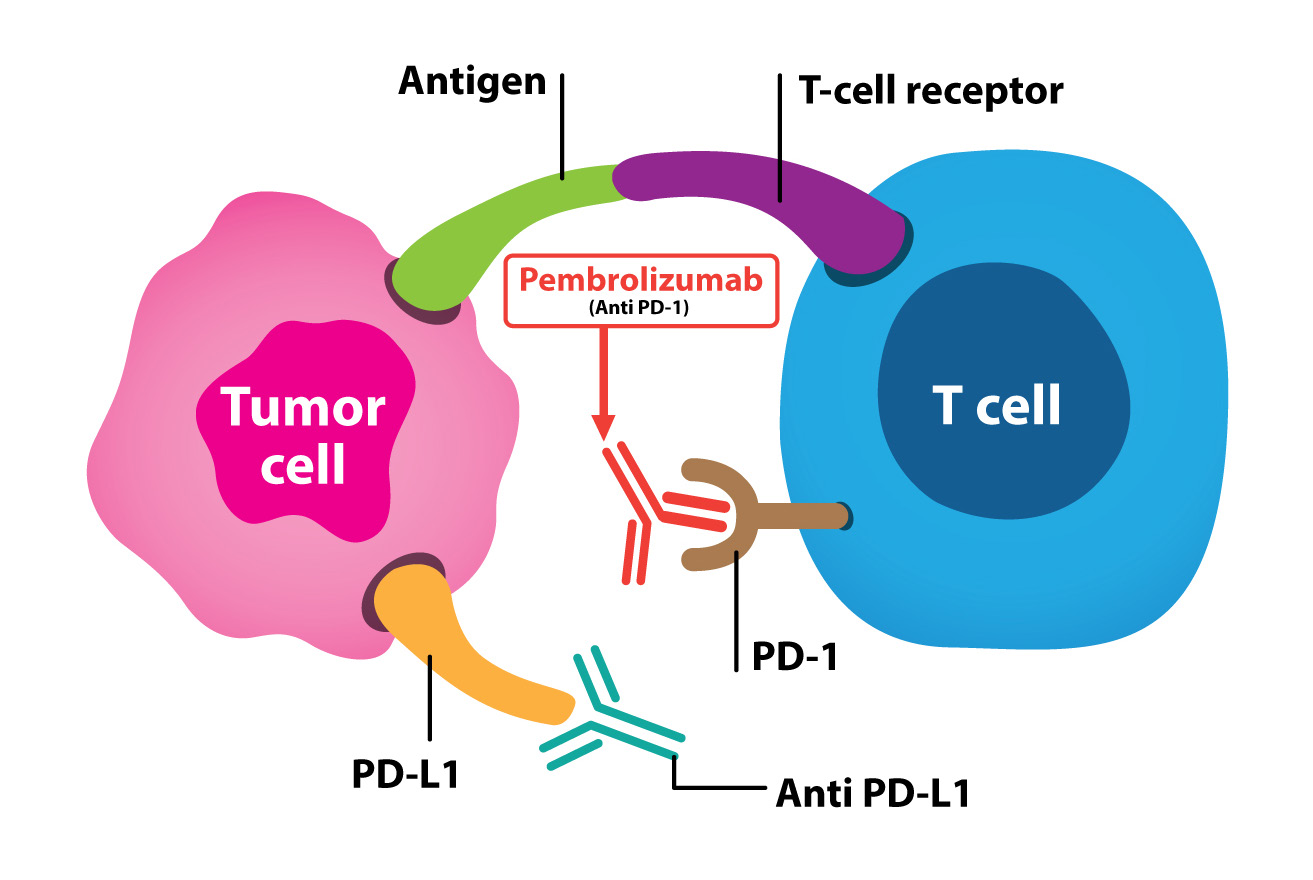
Figure 6: Shows the PD-1 pathway in suppressing anti-tumor immunity.
Metastatic esophageal cancers carry a poor prognosis with limited therapeutic options, and few major therapeutic advances have been made.While improving molecular characterization will continue to identify subsets of patients who may benefit from targeted therapies such as trastu- zumab in HER 2 overexpressed adenocarcinoma,the majority of patients do not yet benefit from these therapies.7 Despite recent improvements in outcomes with trastuzumab and ramucirumab the prognosis for advanced disease remains poor, with a median overall survival of 1 year.
Immunotherapy has caused a paradigm shift in the treatment of melanoma, non-small cell lung cancer, kidney cancer and bladder cancer and its use has been actively investigated in several tumor types. The approaches to utilized immunotherapeutic anticancer effects have come from the development of inhibitory antibodies which modulate immune check points, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed death ligand 1 (PD-L1), and programmed cell death protein 1 (PD-1). Immunotherapeutic mechanisms have been involved in numerous inhibitory and co-stimulatory molecules that interact to form a network of activating and inhibitory pathways which are called immune checkpoints pathways. These serve to regulate the human immune system. This molecular interaction allows for uninterrupted pathogen-fighting capabilities while simultaneously preventing autoimmunity and persistent immune response.8 However, tumor cells frequently escape immune detection by hijacking elements of these checkpoint pathways therefore inhibiting T cell effector function and reduced tumor surveillance and tumor recognition.9 The development of antibodies to immune checkpoints, known collectively as immune checkpoint inhibitors, has now translated to improved patient outcomes in several malignancies.10,11
There has been extensive research regarding the biomarkers to predict who should be responsive to immunotherapy or any additional treatment that should be added to make the tumor respond to the immunotherapy. PD-L1 expression and microsatellite instability are among the early studies for possible biomarker for immunotherapy response. Patients with high PD-L1 expression12 or mismatch-repair deficient, microsatellite instability- high (MSI-High),13 seem to respond well with anti-PD1 blockage regardless of type of tumor. This patient’s tumor has high PD-L1 expression but mismatch-repair proficient, microsatellite instability- stable (MSS) yet still showed a good response with anti-PD1 blockage, pembrolizumab (Figure 6). This patient we report had already been heavily pretreated, in general, we would expect a poor response to chemotherapy. Recent phase Ib trial of pembrolizumab for patients with PD-L1-positive advanced esophageal carcinoma cohort, KEYNOTE-028, revealed 30.4% response rate in the patient’s tumor with a PD-L1 expression ≥1%.14 Given this favourable study result, there is an on going phase III study, KEYNOTE-181, open-label study of second-line pembrolizumab vs single-agent chemother- apy in patients with advanced/metastatic esophageal adeno carcinoma which is expected to have 600 patients enrolled.15
Esophageal cancer is a serious malignancy with regards to mortality and prognosis. Effective treatment is needed to improve patient outcomes. There is ongoing evidence of anti-PD1 blockage which can be used in this cancer but it is very challenging finding the biomarker to determine who should respond to the treatment. Our patient presented as heavily pretreated, with a high PD-L1 expression metastatic esophageal squamous cell carcinama, and showed a very good response to anti-PD1 blockage agent. The multicenter phase III result is currently enrolled and the result is eagerly awaited.