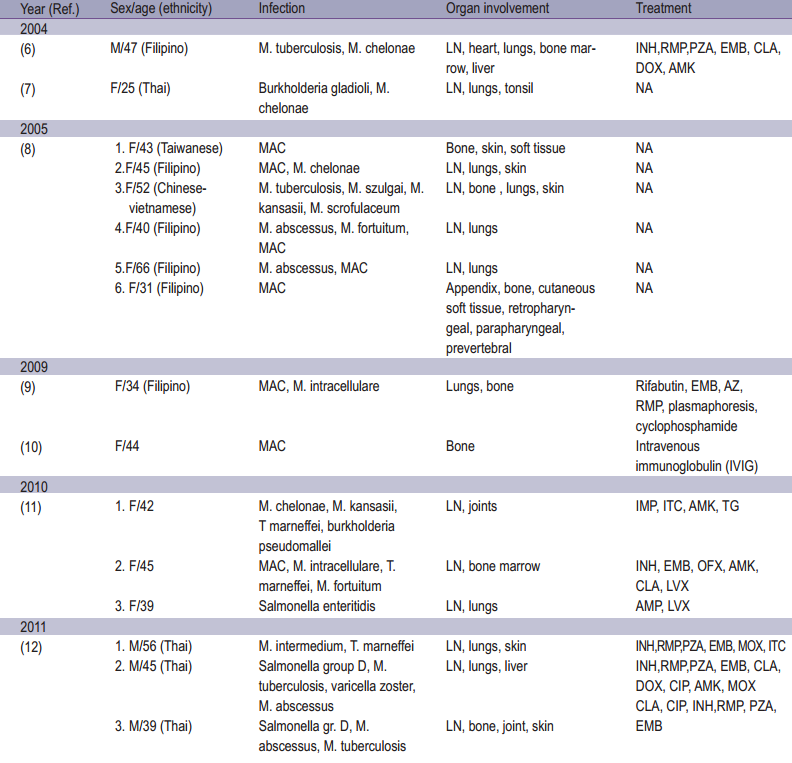
Non-tuberculous mycobacterium (NTM) infection in immuno competent hosts with associated Sweet’s syndrome have been reported in Thailand and Taiwan since 1998.1-5 Adult onset -immunodeficiency syndrome associated with anti-interferon gamma autoantibodies was first described in patients without human immunodeficiency virus (HIV) infection in 2004. The strong inhibitory anti-interferon gamma activity in the patient’s plasma and a high affinity neutralizing anti-interferon gamma were identifed.6,7 It is associated with disseminated infections with lowly pathogenic microorganisms including non-tuberculous mycobacteria (NTM), Salmonella, Burkholderia sp., Talaromyces marneffei, Cryptococcus neoformans, Histoplasma capsulatum and varicella zoster virus.8-23 This condition was predominantly found in South-east Asians.10,12-14,17, 22,24,25 Although it’s rare, most patients developed opportunistic infections without HIV infection emerging as an important cause of morbidity and mortality.5,26
The integrity of interleukin-12 (IL-12) - interferon gamma (IFN-gamma) pathway plays a crucial role in the control of infection with intracellular microorganisms, salmonella and dimorphic molds.27 In normal responses, the activation of phagocytes results in the production of IL-12 and tumor necrosis factor - alpha (TNF-alpha). IL-12 is involved in the production of IFN-gamma by T cells and natural killer (NK) cells. IFN-gamma binds to its receptor, IFNGR, and leads to phosphorylation of JAK2, JAK1 and STAT1, and phosphorylated STAT1 homodimerization. The homodimerization of STAT1 then translocates to the nucleus resulting in upregulated IFN-gamma transcription gene leading to macrophage activation, differentiation and turning to upregulate the expression of IL-12 and TNF-alpha. The activated macrophages are capable of killing intracellular microorganism by the induction of autophagy, nutrient deprivation and exposure to reactive oxygen species and antimicrobial peptides.28,29 (Figure 1)
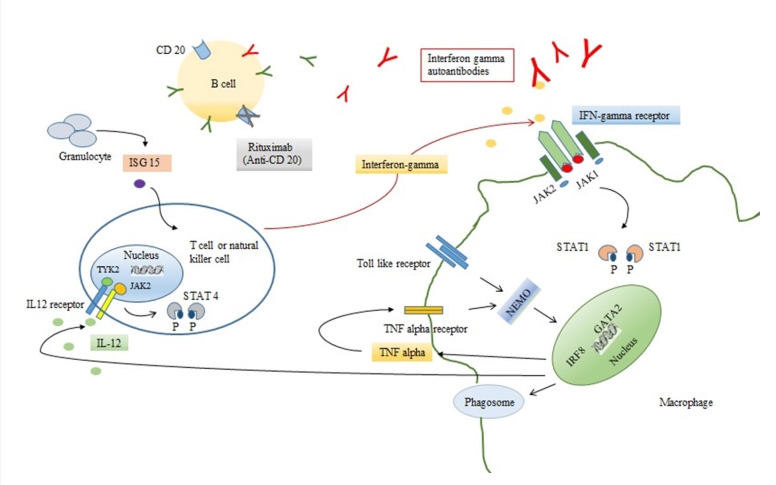
Figure 1: Pathogenesis of interferon gamma autoantibody associated with adult onset immunodefciency syndrome and role of Ritux imab in treatment
Disruption of this pathway, either in primary immunodeficiencies or anti-cytokine antibodies, may lead to susceptibility to such infections.27,30 Mendelian susceptibility to mycobacterial disease (MSMD) is a rare genetic defect which manifests as an autosomal and X-linked mutation. These diseases are characterized by a predisposition to weakly virulent mycobacteria such as bacillus Calmette-Guerin vaccines (BCG-osis), non-tuberculous mycobacteria31 and other intracellular microorganisms such as salmonellosis, histoplasmosis, coccidioidomycosis32-34 which generally occurred at the early age of onset (infancy to early childhood). Mutation of three autosomal genes are implicated in the control of IFN-gamma production including IL12B (encode the subunit of IL-12 and IL-23),35,36 IL12RB1 (IL-12 receptor B1),37,38 and ISG15 (interferon-stimulated gene 15).39 Other autosomal mutation genes implicated in IFN-gamma responsiveness are IFNGR1 (interferon-gamma receptor 1),32 IFNGR2 (interferon-gamma receptor 2),40,41 STAT1,42,43 and IRF8 (interferon regulator factor 8).44 Mutations of IKBKG (encode NEMO) and CYBB (encode gp91phox subunit of phagocyte NADPH oxidase) cause X-linked MSMD.45-47 IFNGR1 and IL12RB1 deficiency account for almost 80% of MSMD patients.48 The prognosis of disease depends on the type and the severity of genetic defects. GATA2 is an essential transcription factor and plays a critical role in early hematopoietic, lymphatic and vascular development.49 GATA2 deficiency has a wide range of clinical phenotypes and varies in the age of onset from early childhood to late adulthood. Most of the patients had profoundly decreased or absence of circulating monocytes, natural killer cells, dendritic cells and B cell with opportunistic infections including human papillomavirus infection, non-tuberculosis mycobacterial, histoplasmosis, cryptococcal meningitis, invasive aspergillosis and Clostridium difficile infection.50,51 In the case of anti-cytokine antibodies, anti-interferongamma autoantibodies were acquired in adult onset with predisposition to NTM infections and other opportunistic infections. The trigger of the production of anti-IFN-gamma autoantibody remains unknown, however, genetic factors are strongly suspected to be involved. A clinical study from Taiwan indicated that HLA-DRB1*16:02 and DQB1*05:02 are associated with this syndrome.52 A recent clinical study in 32 patients from Thailand also demonstrated a strong association between the HLA-DRB1 and DQB1 allele, especially HLADRB1*15:01, DRB1*16:02 and disseminated opportunistic infection with anti-IFN-gamma autoantibody.53 Although the disruption of IFN-gamma - IL-12 pathway increased the susceptibility to similar infections, the age of onset of diseases was useful to discriminate these conditions.
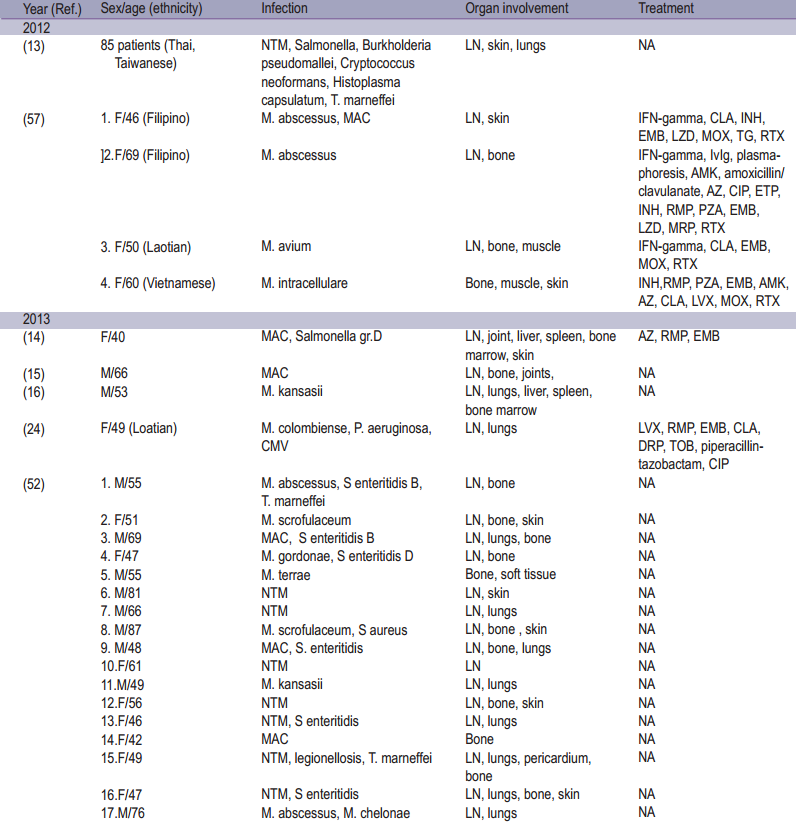
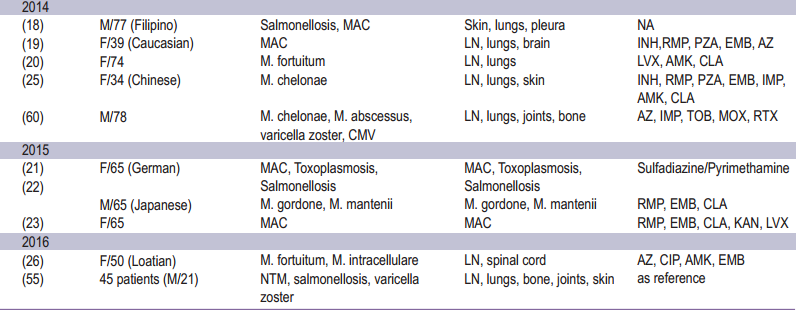
Anti-IFN gamma autoantibodies comprise the majority of anticytokine autoantibodies which are described as a ninth category of primary immunodeficiencies (PIDs) the so called “Phenocopies of PIDs” according to the 2015 International Union of Immunological Society (IUIS) classification.54 This syndrome has been demonstrated to be strongly associated with immunodeficiency leading to opportunistic infection in adults. Most of whom occurred among Asian population (92.2%) occuring more frequently among women.26 The incidence and prevalence may be underestimated, as suggested by several studies describing patients with disseminated NTM infection but for whom Anti-IFN gamma autoantibodies was not investigated.13 The disease typically manifests in adult onset of life (median 48 [range 44.8-60.0] years of age).13,26 From the literature review, non-tuberculous mycobacterium (NTM) was the most opportunistic infection especially rapidly growing mycobacteria and slowly growing mycobacteria.13,26 Other infections were reported including Herpesviridae reactivation, Salmonella, Talaromyces marneffei, Cryptococcus neoformans, Histoplasma capsulatum, Burkholderia pseudomallei and varicella zoster infection. 8,12,13,17,26,52,55 Most patients manifested with multiple organs involvement. Lymph nodes were the most common sites of involvement, followed by the osteoarticular tissue, lungs and skin, respectively. Cervical lymphadenopathy was the most common manifestation.13,55 Reactive dermatoses were reported including Sweet’s syndrome, erythema nodosum, lobular panniculitis, and generalized pustular eruption (acute generalized erythematous pustulosis, pustular psoriasis and subcorneal pustulosis). Among these, Sweet’s syndrome was frequently linked to anti-IFN gamma autoantibodies.56 The epidemiology of adult onset immunodeficiency with anti-interferon-gamma autoantibodies is summarized in Table 1.
Table 1: Epidemiology of adult onset immunodefciency associated with Anti-interferon-gamma autoantibodies
In general, for the diagnosis in adult onset of immunodeficiency associated with anti-IFN-gamma autoantibodies, HIV testing should be done in all patients. Lymphocyte phenotype might be useful to differentiate GATA2 deficiency as it demonstrated a low number of NK cells, monocytes and B cells.
In almost all patients, anti-IFN gamma autoantibody in plasma was detected and demonstrated the inhibition of STAT-1 phosphorylation function.12,13,57 Indirect enzyme-linked immunosorbent assay (ELISA) was applied to detect the Anti-IFNgamma autoantibody in plasma or serum of patients. The higher titers of autoantibodies (≥ 10-5 dilution) tend to determine the recurrent episodes of NTM infection.55
Inflammatory markers which indicated active disease activity including a high proportion of white blood cell counts, absolute neutrophil counts, ESR, CRP and alkaline phosphatase level.17,55 The IFN-gamma autoantibodies titers was relatively higher in active infection compared to those without infection.17
A lymphocyte phenotype study revealed an increase in natural killer cells and activated macrophage (CD119) counts and a drop in naive T lymphocyte counts in this syndrome compared to healthy cells, whereas the total numbers of the CD4+, CD8+, CD3+ T and CD19+ B cells were similar.13,58,59
Imbalance of Th1/Th2 in Anti-IFN gamma autoantibodies was demonstrated in several reports. Patel et al.,8proposed that these autoantibodies are able to block the binding of IFNgamma and to inhibit some downstream cytokine production resulting in the reduction of TNF-alpha and IL-12.In the study of the intracellular cytokine production of T cell, upon the intact T cell receptor (TCR) activation, T cell proliferation and IL-2 receptors of patients, IFN-gamma and TNF-alpha (Th1) production were upregulated, nevertheless the production of IL-4 (Th2) and IL-17 (Th17) remained normal in comparison with healthy cells.59 In yet another study, there was a discrepancy reported in the decline of production of TNF-alpha and IL-2.58
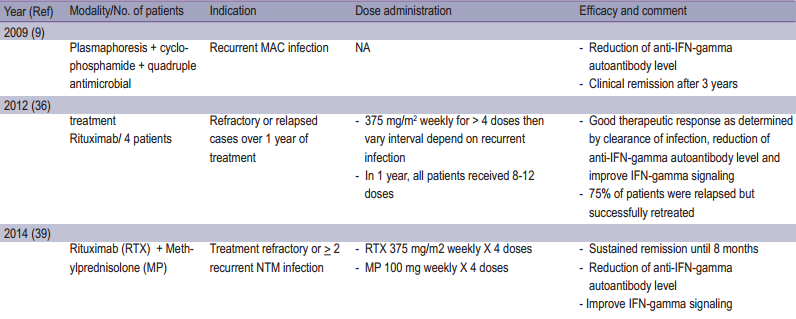
Specific treatment for adult onset immunodeficiency associated with IFN-gamma autoantibody is not codified. Despite intensive treatment with antimicrobial therapy, more than half of these patients still had persistent NTM infection and required long term treatment.26,55
Rituximab (Anti-CD20), has been used off-label in progressive refractory patients associated with IFN-gamma autoantibodies, demonstrated the good clinical response, decline in IFN-gamma autoantibodies titers as well as neutralizing ability.57,60 Other strategies included plasmapheresis, cyclophosphamide,9 IFN-gamma and intravenous immunoglobulin (IVIG).10 (Table 2)
In our opinion, immunomodulator or immunosuppressive drugs seem to play a role in cases of refractory or relapse infections. Further investigations for modalities of treatment are needed to optimize the clinical outcome.
Table2: Summary of reported treatment for adult onset immunodefciency associated Anti-interferon-gamma autoantibodies.
More than half of anti-IFN-gamma autoantibodies patients had persistent or relapsing infections, only 20-30% were declared cured and there was a 10% mortality rate.26,55
Adult onset immunodeficiency associated with anti-IFNgamma autoantibodies was predominantly found in South East Asians including Thai patients. Most patients manifested as NTM infection with multiple organ involvements especially cervical lymphadenopathy, however, other opportunistic infections such as salmonella, fungus and virus can occur. Physicians should be aware of this syndrome. Despite intensive antimicrobial treatment, some patients’ symptoms remained persistent or the infection relapsed. Other therapeutic targeting antibody production or targeting IFN-gamma autoantibodies may warrant further investigations.