Cases of fulminant hepatitis caused by herpes virus infection is quite rare.1 Most reported cases are pregnant woman and immune suppressed patients such as renal transplantation patients and patients on prolonged steroid therapy.2-6 Most cases of herpes infection leading to fulminant hepatitis, are post mortem diagnosis.1 Early diagnosis and treatment can save the patient’s life and avoid the need for liver transplantation.
Here we report on an immunocompetent patient who developed fulminant hepatic failure caused by herpes simplex type I infection. Although the patient came to our hospital presenting an infection after seven days of illness, early diagnosis and treatment saved his life and prevented fatal liver failure. We demonstrated the use of real-time polymerase chain reaction (PCR) as a useful tool in therapeutic monitoring of herpes simplex virus (HSV) type I clearance. By using the PCR threshold cycle (Ct), we could show that the HSV infection clearance was quite slow from the serum results. Further studies should be investigated to identify the optimal dose and duration of the administration of acyclovir therapy in this setting.
A 47-year-old man presented with a previous history of good physical health. He had been living with diabetes mellitus (DM) type 2 for years without obvious DM complications. His HbA1C tested one month prior to admission was 5.7 mg/dl. He is an occasional alcohol drinker. He presented with symptoms of mild sore throat and fever. He sought treatment in a private clinic and was treated for acute pharyngitis with amoxillin/clavulanic acid. Three days later, he was admitted to another hospital due to persistent fever accompanied with increased malaise and anorexia. Further investigations indicated a possible case of Ebstein Barr viral (EBV) infection. Four days later, he was transferred to Bangkok Hospital for further evaluation and proper management.
A physical examination was undertaken on arrival at Bangkok Hospital at 15:00 on August 29, 2013. The patient presented as febrile (body temperature 38.5°C), no dyspnea but looked weak and was somnolent, yet conscious.
His heart rate was 86/min at rest, with a respiratory rate of 20/min and his blood pressure was 145/80 mmHg. He was not pale and showed no icteric sclera. The pharynx was not infected and there was no exudate on either tonsil. Chest and abdominal examinations were unremarkable. There was no stigma of chronic liver disease observed. No skin blister lesions were found. From the physical findings, no active focal infection was detected.
Investigations and results
The significant abnormal liver function test (LFT) revealed a high elevation of transaminases (ALT 2561 U/L, AST 3209 U/L), total bilirubin 1.2mg/dl, direct bilirubin 0.9 mg/dl, total protein 6.38g/dl, albumin 3.71g/dl and prolonged prothrombin time (PT) 14.2 seconds (sec) (the normal range is 10.5-13.4sec). Complete blood count (CBC) revealed Haemoglobin (Hb) 16.6g/dl, leucopenia with total white blood cell count (WBC) 2,510 cells/mm2 (polymorphonuclear cell (PMN) 62.9%, lymphocyte 16.5%) and a platelet count that dropped to 92,000 cells/ mm3. These results indicated that the patient was suffering from acute fulminant hepatitis with impending liver failure. Therefore a working diagnosis for etiologic agents causing fulminant hepatitis was investigated.
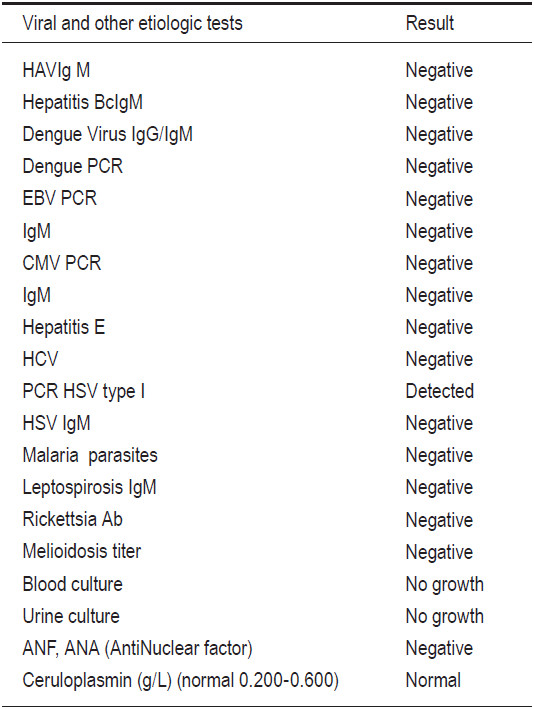
The patient was treated with empirical antibiotics while investigations took place, and the liver transplantation team was informed to prepare a liver transplantation if required. The patient was suspected to be suffering from EBV infection with complicated and progressive liver failure. The hematologist performed a bone marrow biopsy, and the result ruled out infections associated with hemophagocytosis syndrome and other hematologic diseases. On admission day 2, he became more confused, accompanied with blurred consciousness and a persistent high intermittent fever of 39-40°C. We transferred him to the intensive care unit for closer observation so the patient could receive 24 hours care. The follow-up blood chemical tests and LFT of the patient (Table 1) showed a marked increase in transaminase and progressive jaundice. He also developed acute pancreatitis. Computer tomography of the abdomen revealed no significant abnormality of the liver gall bladder and the common bile duct apart from a mild swelling of the pancreas and spleen. At around 48 hours after admission, we received a positive identification of the PCR-Herpes Simplex virus (HSV) type I while all other viral studies were negative (Table 2).
Table 1: Results of blood chemistry and transaminase.
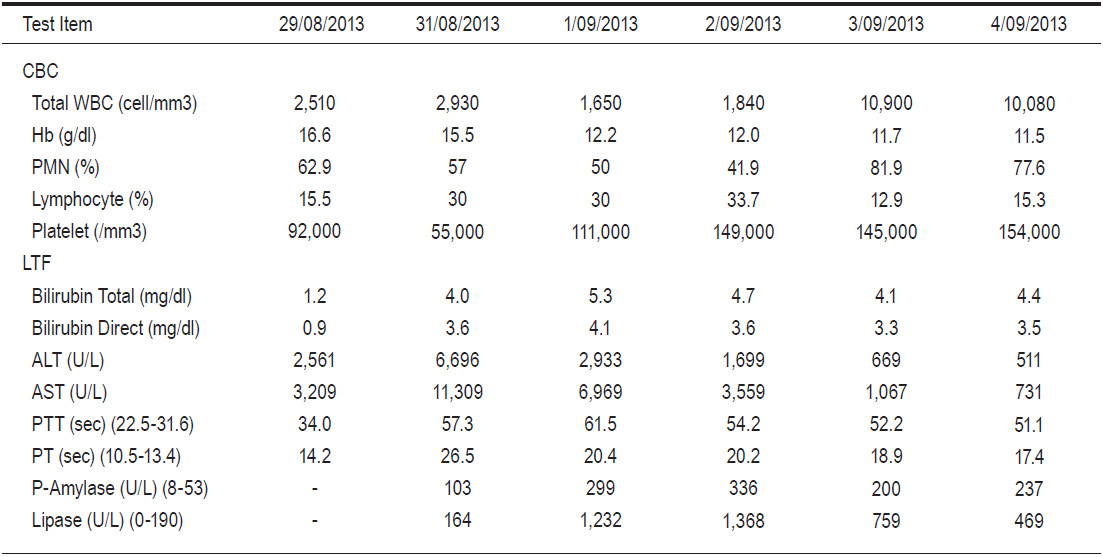
Table 2: Initial results of viral and other etiologic tests that may be a cause of fulminant hepatitis.
We started acyclovir therapy on August 31, 2013 at 9.00 am (around 48 hours after admission). We observed that his clinical outcome slowly improved. Fever presented up to 12 days after the medication was administered, while the transaminase and bilirubin levels gradually improved. The patient responded slowly and clinical intermittent fever and HSV type I was still detected in blood samples, so we applied our in-house laboratory real-time PCR to monitor his treatment.
Monitoring the Herpes virus during therapy
The quantitative real-time PCR for Herpes virus is not commercially available for monitoring the viral load as in the case of other viruses. We developed a real-time PCR based on SYBR Green I chemistry for compara- tive quantification of the Herpes Simplex virus. The key conceptual innovation to the quantification in real-time PCR is the threshold cycle (Ct), that is, the cycle at which the fluorescence signal from the PCR reaction exceeds the baseline fluorescence. The Ct is dependent on the amount target present at the beginning of the reaction; the fewer cycles it takes to reach a detectable fluorescence the greater the initial copy number. With most real-time PCR machines it takes about 1010 copies of PCR product to produce a significant signal. The equation Nn = No (1+E)n predicts that if there are 1,000,000 copies at the beginning of the PCR reaction, the instrument will detect a signal around cycle 14.
If the starting copy number is 100,000 the first signal will appear around cycle 17. A serial tenfold dilution of standard levels shows that the Ct value differs by 3.3 for each dilution.7,8 We can assume on this basis that if the virus responds to the therapy regimen, the Ct of consecutive samples should rate higher than previous samples, which means in should be that the viral load is decreasing or responding to therapy.
Clinical Specimens and DNA extraction
A total of seven consecutive whole-blood specimens were collected during the clinical course and therapy intervention from September 4, 2013 to October 12, 2013. The nucleic acid was extracted from 0.2ml of plasma by using MagnaPure Compact Nucleic Acid Isolation Kit I according to the manufacturer’s instructions. DNA was eluted in the final volume of 50μl of elution buffer and was stored at -30oC until used.
Comparative Quantification of HSV DNA with Real-time PCR
The PCR primers HSV3 (5’-gcg ccg tca gcg agg ata ac-3’) is a common forward primer for Herpes Simplex virus type 1 and 2 (HSV1, HSV2), primer HS1 (5’-ggg gta ctt aca gga gcc ctt-3’) is a specific reverse primer for HSV1, primer HS2 (5’-gcc ctc ttg gta ggc ctt c-3’) is a reverse primer specific for HSV2. Five μl of extracted DNA from each sample was mixed with 0.4 μM of forward primer and 0.8μM of reverse primer in a 20μl reaction volume using a SensiFAST SYBR No-ROX Kit (Bioline Reagents Ltd. United Kingdom). The samples were amplified in a Light- Cycler Nano Real-time PCR system (Roche Diagnostic,USA). The real-time PCR condition consisted of a two steps cycling of 95oC for 10 seconds and 60oC for 30 second for 45 cycles followed by melting analysis from 65oC to 95oC. For each consecutive sample the previous DNA sample was repeated in the same run to check the stability of the DNA sample and for comparison with later samples.
HSV1 was detected from each sample according to the date of serum sample and the Ct of the first serum collection on September 4, 2013 was 16.20 and the Ct values increase in consecutive serum samples (Table 3 and Figure 1). With the last serum sample taken on October 12, 2013 the Ct value was 37.66 which is not a signal from a specific PCR product because the melting temperature (Tm) of the PCR product is 85.76oC. The HSV1 specific PCR product has a Tm around 87.7-88.0oC (Table 3, Figure 2). The PCR products were verified by agarose gel electrophoresis to check for a PCR product size of 152 bp. (data not shown). The real-time PCR assay using specific primers for HSV2 were negative for all samples tested. The results showed that the Ct increased in the consecutive samples which meant that the amount of HSV1 virus was decreasing probably from therapeutic intervention and the virus was cleared from circulation as a PCR result on October 12, 2013. From this study, the qualitative real-time PCR can be used to monitor the virus in circulation by using a comparative PCR between consecutive samples. According to the result of the PCR HSV-threshold cycle (Ct), we continued therapy with Acyclovir intravenously for 2 weeks and followed by oral Valacyclovir up to 6 weeks until the negative test result of October 12, 2013. The patient returned for a follow-up on December 15, 2013, with almost a full physical recovery. The liver transaminases returned to nearly normal levels as follows: ALT 49 U/L, AST 35 U/L, Total bilirubin 1.3 mg/dl and direct bilirubin 1.0mg/dl.
Table 3: The Ct value from consecutive samples show that the viral load from the
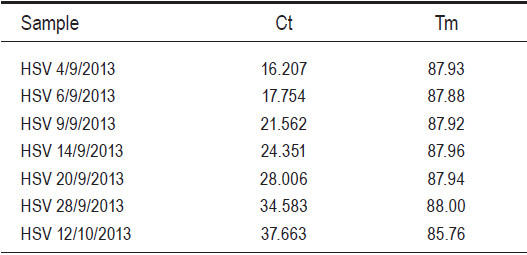
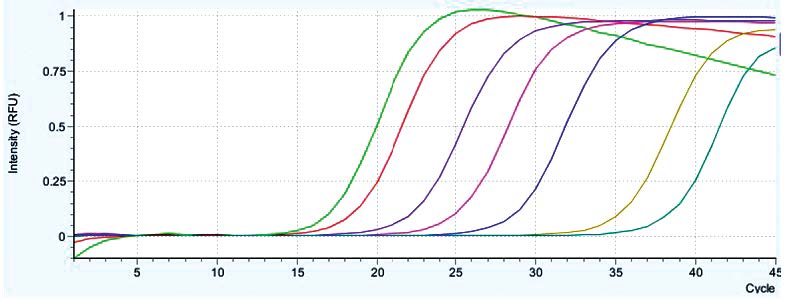
Figure 1: The amplification plot of real-time SYBR Green I PCR assay for Herpes Simplex type 1 from consecutive serum samples from a fulminant hepatitis patient. (The light green is the sample from 4/09/2013, the red from 6/09/2013, the purple from 9/09/2013, the pink from 14/09/2013, the blue from 20/09/2013, the yellow from 28/09/2013 and the green from 12/10/2013).
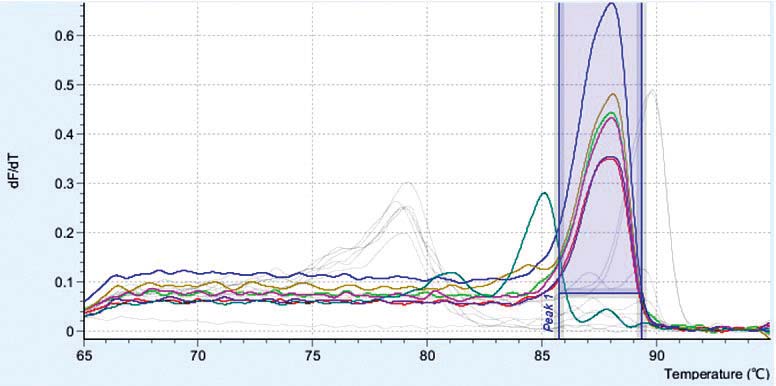
Figure 2: The melting curve profiles of HSV1 real-time PCR. The HSV1 PCR products have a Tm around 87.8-88.0 but the primer dimer product has a Tm of around 85.7 which is clearly separate from the HSV1 PCR product.
Herpes Simplex virus (HSV) typically causes muco- cutaneous vesicular oral (herpes labialis) or genital lesions (herpes genitalis); visceral involvement may occur in some clinical settings. HSV hepatitis is rare and accounts for only 1% of all acute liver failure (ALF) cases and only 2% of all viral causes of ALF.1 HSV hepatitis is one of several clinical manifestations of HSV sepsis or disseminated disease leading more frequently to encephalitis, pneumonia and esophagitis2, which mostly affects immunocompro-mised patients such as organ transplant patients3-4, pediatric patients5 and patients in the third trimester of pregnancy6, but there have been reported cases of up to 25% in immunocompetent patients as well.9 Both HSV-1 and HSV-2 have been implicated as etiologic agents. Compro- mised cellular immunity is a major risk factor for HSV sepsis, either as a primary infection or as a reactivation of occult chronic HSV infection. HSV hepatitis after liver transplantation and heart transplantation has been reported. HSV reactivates after transplantation in approximately 60% of recipients not given antiviral prophylaxis.
Reactivation of HSV usually occurs during the first 1-2 weeks after transplantation.10 HSV infection of neonates is uncommon. The three major forms of neonatal HSV infection are disseminated disease (25%), central nervous system disease (30%) and skin, eye, and mouth disease (45%). Death from disseminated disease is usually caused by severe coagulopathy; and extensive hepatic and pulmonary involvement.11 Around 2% of women acquire HSV during pregnancy. Changes in the immune system during pregnancy make pregnant patients more susceptible to acute HSV hepatitis, with a 40% risk for HSV-related ALF and death. Humoral and cell-mediated immunity are most depressed in the third trimester, as demonstrated by a decreased T-cell count and altered B-/T-cell ratios. In immunocompetent hosts, however, only primary infections have been associated with hepatitis. Severe HSV hepatitis in immunocompetent patients is a very rare condition, but it may lead to fulminant deterioration of liver function and can be rapidly fatal. HSV hepatitis is often left undiagnosed due to the absence of specific signs or symptoms, which include flu-like illness, fever and abdominal discomfort. Mucocutaneous lesions are only present in up to 50% of cases.12
Typically, anicteric hepatitis is seen in patients with HSV hepatitis. Anicteric hepatitis refers to a liver profile showing a significant increase in transaminases (100-1000 fold) with a relatively normal or low bilirubin. There may be a marked elevation of AST greater than ALT.13 Mild, asymptomatic liver enzyme elevations may be seen in 14% of immunocompetent patients with acute genital HSV infection. Severe HSV hepatitis is usually marked by significant elevations in transaminases, hyper-bilirubinaemia and coagulopathy. Disseminated intravascular coagulopathy (DIC) is frequently reported, and encephalopathy is a late sign of the disease.
A total of 137 cases (132 from literature, 5 institutional) of HSV hepatitis were reported. The main features at clinical presentation were fever (98%), coagulopathy (84%), and encephalopathy (80%). Most cases (58%) were first diagnosed at autopsy and the diagnosis was suspected clinically prior to tissue confirmation in only 23%. The course of the disease is often rapid and frequently fatal. The mortality rate can be as high as 75-90%, mainly because of delayed diagnosis and treatment with antiviral therapy.9
The diagnosis of HSV hepatitis should be considered in any patient with acute hepatitis, particularly with fever, leukopenia, and a negative hepatitis serology for hepatitis A, B, C, D, E, EBV and CMV especially when DIC is present and liver failure is suspected. Viral serology and cultures are extremely sensitive and can be used as a screening tool but are very poorly specific. Rapid viral culture is available for HSV which shortens the time for isolation to 4 days. Detection of HSV DNA by PCR appeared to be more discriminating than serological testing for diagnosing or excluding HSV as a cause of ALF. All HSV hepatitis cases had high DNA levels, supporting the use of HSV PCR as a screening test for indeterminate ALF to formulate a rapid management plan versus other inaccurate and invasive tests, such as serology and liver biopsy.14
Although not always possible due to coagulopathy, the gold standard for diagnosis of HSV hepatitis is liver biopsy. Histology shows extensive areas of hepatocyte necrosis with adjacent congestion but minimal inflam- matory infiltrates (Figure 3).13 Cowdry type A inclusions, nuclei with large eosinophilic ground glass-like inclusions surrounded by a clear halo, are pathognomonic for HSV hepatitis. Immunohistochemical staining can be done to confirm the diagnosis, and the presence of viral antigens can be demonstrated by immunoperoxidase staining and by identifying monoclonal antibodies against HSV antigens (Figure 4).13
HSV associated fulminant hepatic failure carries a high mortality risk, early intervention with acyclovir has been shown to be life-saving.15 Norvell JP, et al reported9 in HSV hepatitis cases, 49 (36.6%) of 134 patients received acyclovir treatment. Patients who received treatment were less likely to die or require liver transplantation (51% vs. 88.1%, p < 0.001) compared to untreated patients. In treated patients, the mean time from overt symptoms to treatment with acyclovir was 4.2±1.8 days. There was a delay in the initiation of treatment (mean 4.7 vs. 3.5 days, p = 0.03) in patients who died or required liver transplantation as compared to patients who survived. Variables on presentation associated with death or need for LT compared to spontaneous survival: male gender, age > 40 year, immuno- compromisedstate, ALT > 5,000 U/L, platelet count< 75x103/L, coagulopathy, encephalopathy, and absence of antiviral therapy.9 Therefore, it is a generally accepted consensus to begin antiviral therapy pre-emptively with acyclovir in cases of acute liver failure of unknown origin.
Acyclovir is classified as a category B drug in pregnancy with no fetal risk demonstrated in animal or human studies. High-dose intravenous acyclovir (at least 10 mg/ kg every eight hours) is effective and appears to be safe in pregnancy. The safety of acyclovir in pregnancy has been reported in multiple studies where the rates of birth defects (2.6%) were not different from the expected rate (3.2%) in the general population.16 The current treatment recommendation for fulminant HSV hepatitis in pregnancy is intravenous acyclovir, with the addition of foscarnet for acyclovir-resistant cases.17 Therapeutic plasma exchange (TPE) has been reported to treat post-partum HSV-related ALF patients. TPE may have a therapeutic role in acute inflammatory disorders by reducing viral load, attenuating systemic inflammation and liver cell injury. However, further investigation is needed to clarify this potential role.18
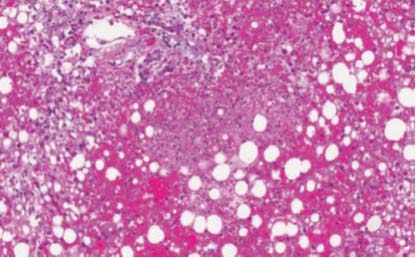
Figure 3: Liver pathology of HSV hepatitis: Zones of hepatocyte necrosis surrounded by hemorrhage without significant inflammation [100x magnification; Hema- toxylin-Phloxine-Saffron (HPS) stain].13
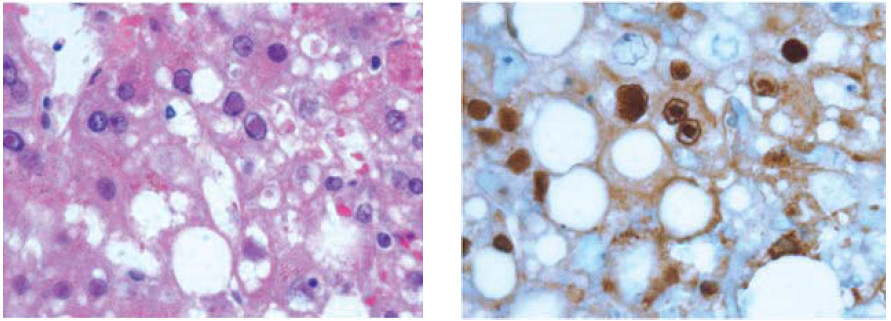
Figure 4A-B: HSV inclusions in infected hepatocyte.13 (A)Viral inclusions are readily visible in infection hepatocytes. (600x magnification; Hematoxylin-Phloxine-Saffron (HPS) stain). (B) Immunohistochemistry for HSV virus highlights viral inclusions (600x magnification).
High urgency liver transplants should also be considered early in the course of the disease as it has been shown to improve outcomes. So far, 148 HSV-related ALF cases have been published, of which 9 underwent liver trans- plantation (LT). The reported post-transplant survival is poor, with over 60% dying in the first year.9 Dosing and duration of antiviral therapy after LT are not established. Quantitative HSV DNA testing is useful in predicting morbidity and mortality after LT and in determining the optimal type and length of antiviral regimen to use post- transplant. Because of the risk of recurrence, life-long prophylaxis with acyclovir is recommended.19
Moldovan B, et al.20 based on the Scientific Registry of Transplant Recipients registry (USA), reported a better recovery in children than in adults. During the study period (1985-2009), 30 patients were listed for HSV hepatitis: 7 recovered spontaneously and 5 died, prior to transplantation. The remaining 10 children and 8 adults were transplanted. The chance of recovery was significantly higher in children than in adults (7/19 vs. 0/11, p = 0.02). In children, survival was similar between HSV patients and the matched controls (5-year survival: 69% vs. 64%, p = 0.89). Conversely, survival was poor in adult HSV (5-year survival: 38% vs. 65%, p = 0.006), with 62% of them dying within the first 12 months.20
HSV hepatitis represents a broad spectrum of disease from mild aminotransferase elevation to fulminant liver failure and death. HSV hepatitis should be considered in patients with fulminant liver failure of unknown cause. Acyclovir given in the early stages of fulminant hepatic failure may prevent mortality and avoid the need for liver transplantation. At an advanced stage, liver transplantation should also be considered. HSV DNA testing is useful in predicting morbidity and mortality after LT and in determining the optimal type and length of antiviral regimen to use post-transplant.