
Located on the basement floor of the Wattanosoth Building, you will find many highly specialized technical medical equipment. For this is where the Department of Oncology Imaging and Nuclear Medicine, and the Department of RadiatioTherapy are located. Both of these departments are part of Wattanosoth Cancer Hospital. Her Royal Highness Princess Maha Chakri Sirindhorn bestowed the name of “Wattanosoth” to the hospital in 2006. The meaning of Wattanosoth is “hospital for advanced medicine”.
Wattanosoth Cancer Hospital is entirely devoted to provide cancer patients with the most effective range of traditional and innovative cancer treatment available. It is Thailand’s first private hospital devoted entirely to the treatment of cancer. The hospital is committed to improving the quality of life of cancer patients and survivors and to share research with fellow scientists, professionals, students and the community at large.
Bangkok Hospital set up a project management team which consisted of several specialists in the medical field. The process for procurement of medical equipment ran parallel to the design of the new building in 2004. The goal was to have services available for patients in late 2005.
The most expensive and complicated medical equip- ment that Bangkok Hospital invested in at the time were the PET/CT, Cyclotron and Novalis. These now sit on the basement floor of Wattanosoth Building. Novalis is a premium BrainLab technology for radiosurgery and stereotactic body radiation therapy (SBRT). The instal- lation of the Novalis was completed in late 2005. The Novalis was first used in the treatment of patients in October 2005.

PET/CT is nuclear imaging technology. It requires the administration of a PET radiopharmaceutical. The most commonly used PET radiopharmaceutical is the F-18 FDG (Fludeoxyglucose) which is a cyclotron-produced isotope with a half-life of 110 minutes. At that time, there were no medical cyclotrons available in Thailand. So in order to provide PET examinations for patients, a cyclotron and hot lab was needed to produce PET radiopharmaceuticals.
The sourcing process began in mid-2004, and the winning company was instructed to provide a cyclotron, synthesis modules, and quality control equipment tools and design the facility according to internationally rec- ognized standards. Bangkok Hospital agreed to purchase cyclotron and radiopharmaceutical equipment from Supreme Products Ltd. The agreement also included responsibility for overseeing the production of PET radiopharmaceuticals, and the management of radiophar-maceuticals production and after sale services.
After having selected the company for the cyclotron and hot lab project, the contract was signed in January 2005. The contract included the placement of on-site specialist for 6 months to facilitate knowledge transfer after the completion of installation and commissioning. Supreme Product Ltd sourced a cyclotron from the Advanced Cyclotron System Inc (ACSI), Vancouver, Canada (previously EBCO). It is a TR-19 PET cyclotron which can provide a protons beam of up to an energy emission of 19 MeV. The synthesis module for the FDG production was also sourced from ACSI. Hot cells were sourced from TEMA Synergy, Italy.
There were 2 phases for the installation of the cyclotron and hot lab. The first phase included the produc- tion of FDG. The second phase was for the production of other PET radiopharmaceuticals such as C-11 acetate which was planned to take place for about a year after the first phase was completed. Most of the equipment for the cyclotron and hot lab arrived at the hospital on April 20th, 2005. The construction of the hospital was still underway at that time. The Cyclotron was shipped from Vancouver, Canada. Hot cells and radiation produc- tion equipment were shipped from TEMA Synergy, Italy. Since the hospital was keen to commence services using the PET/CT before the end of 2005, the hot cells needed to be shipped by air freight. This was extremely costly. Supreme Product Ltd also needed to provide equipment for quality control tests of the radiopharmaceuticals. Most of the equipment for the quality control (QC) such as the High Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), and Thin layer chromatography were ordered from local suppliers in Thailand.

Figure 1: The installation site for cyclotron.

Figure 2: The TR-19 PET cyclotron.

Figure 3: Installation of hot cells in the hot lab.

Figure 4: Equipment in the quality control room.
The installation of the cyclotron and hot lab took around three to four months. Figure 1-2 and 3 show the cyclotron and hot lab being installed. Figure 4 shows the quality control equipment in the QC room. The entire cyclotron and hot lab system was installed and commis- sioned in November 2005. The first volunteer PET/CT patient was scanned on November 24th 2005.
Establishing a PET and Cyclotron Center is a very complicated project. It needs a lot of effort, dedication, excellent planning, with specialist advice from highly qualified personnel. Many experts in the field of nuclear medicine in Thailand such as Associate Professor Dr. Anchali Krisanachinda, Associate Professor Dr. Rujaporn Chanachai were consulted at the time. A specialist, Professor John Wilson, spent a month here helping to set up the cyclotron, the hot lab and the production of radiopharmaceuticals. Professor John Wilson is a PET radiopharmaceutical expert and facility manager at the Edmonton PET Center at the Oncologic Imaging Depart- ment of Oncology, University of Alberta, Cross Cancer Institute, Edmonton, Alberta, Canada.
To start with, Wattanosoth Hospital outsourced Supreme Product Ltd to manage the production of PET radiopharmaceuticals. Since this was the very first PET and Cyclotron Center in Thailand, not many people had the technical capability to handle this delicate equipment. All of the people who worked on this project were very new to the technology at that time. During the first couple of months, there were a lot of problems related to the failure of FDG production and to the delay of FDG production. The causes of the problems included: lack of experienced production staff and cyclotron engineers, an uncontrolled facility environment dealing with variations of temperature, humidity, the temperature of chilled water, and the electricity etc. The equipment downtime was very high since the engineers did not have enough knowledge and experience to handle cyclotron and hot lab equip- ment. The stock management was also an important issue because most of the materials and supplies needed to be imported from outside Thailand.
In the end, Wattanosoth Hospital took over full management of PET radiopharmaceutical production by mid- 2006. A lot of changes have taken place since the hospital took over the management of PET radio- pharmaceutical process. The hospital has hired engineers, chemists, and a pharmacist to form a PET radiopharma- ceutical production team and arranged the necessary training for them for both on-site training and abroad. Monthly meetings with hospital mechanics and engi- neers were arranged to solve any problems related to facility environment such as room temperature, humidity, electricity, and chilled water system etc. Another FDG synthesis module was purchased as a back-up system in 2006. To improve the quality of the radiopharmaceutical production, a standard for radiopharmaceutical production needed to be applied. In 2007, the hot lab was renovated to make it meet international clean room standards and to meet the international standard for a PET radiopharma- ceutical production facility. A system to monitor room temperature, humidity, and pressure was installed. The production team has implemented many continuous quality improvement projects to improve production processes and to cut costs.
In June 2009, the module for the C-11 acetate was installed. There were many problems during the develop- ment of C-11 acetate. The most severe problem was the leakage of radioactive gas when the production failed. Expert advice from many facilities was consulted. A system to contain radioactive gas was applied. The service for C-11 acetate PET was made available in August 2009.
The project for C-11 PIB (Pittsburgh Compound B) PET for the diagnosis of Alzheimer’s disease was initiated in 2010. The synthesis module for C-11 PIB production was purchased from iPhase Company in Melbourne, Australia. The installation of the module was finished in May 2011. The first volunteer patient for C-11 PIB PET scan was on May 24th, 2011. Production of C-11 radio- pharmaceutical is more complicated than the production of FDG because it is a gas phase production. A lot of caution concerning the potential contamination of cold C-12 in the product needs to be taken. There were many problems to solve, and a lot of experienced technical personnel had to be involved. The C-11 PIB PET scan is the only one of its kind available in Southeast Asia. With such a very short half-life of C-11 (20 minutes), the C-11 PIB has to be produced on-site, close to PET scanner room. There were many problems related to the production, namely the contamination of C-12 in the system which resulted in low specific activity of the product, the man- agement of supplies of material and chemicals for the production, and the leakage of C-11 radioactive gas, etc. It took the team almost a year to gain enough experience in the C-11 PIB production process to make the produc- tion stable.
In 2012, there was demand for an FDOPA-PET for the diagnosis of Parkinson’s Disease. Wattanosoth Hospital decided to invest in FDOPA production in November 2012. The synthesis module for FDOPA was purchased from Trasis, Belgium. The facility preparation, including the gas leakage system and an additional hot cell installa- tion were completed in June 2013. The synthesis module was installed in July 2013. Due to problems with the supply of necessary chemicals, and many problems related to production, the project was delayed for several months. Finally, the FDOPA PET was opened for patient service in January 8th, 2014.
The PET/CT project was also quite a difficult task for the team. The PET/CT at Wattanosoth Cancer Hospital is the first PET/CT in Thailand. It was a very new and very sophisticated technology at that time. Not many people in the medical field were familiar with the technology. Many members of the general public had never heard of PET/CT. A medical team for PET/CT was put together for the PET/ CT project at Wattanosoth Hospital during the installa- tion of the PET/CT in mid-2005. The team had to plan for training and education for team members as well as raising awareness of all related medical staff, marketing staff and the general public. Morning briefings were scheduled every day between the management team, support team and medical team to keep everyone informed about the ongoing process and any problems to resolve.
The PET/CT was installed in the basement of Wattanosoth Building while the building was still under construction. The plan was to launch each service simultaneously to complete cancer care management for our patients. PET/CT was one of the services that was planned to open for service together with other depart- ments at Wattanosoth Cancer Hospital. While the building was still under construction, the team was working at the Bangkok Hospital building. In September, some areas in Wattanosoth Building were open for staff to work. Many documents and related medical equipment needed to be prepared to prepare for the opening of the PET/CT service. The facility also had to be prepared according to a radiation safety program. Staff members were also trained in risk assessment and in the safe use of radioactive substances.
In terms of PET/CT installation and commissioning, a medical physicist had to check and calibrate the system working with engineers from abroad. An expert medical physicist, Assistant Professor Dr. Napapong Pongnapang from Mahidol University, was invited to assist with the acceptance test for the PET/CT. The acceptance test for PET/CT was assessed according to NEMA standard and it took about a week to finish. The team worked very hard to make the PET service available as quickly as possible and the first volunteer PET/CT patient was on November 24th, 2005.
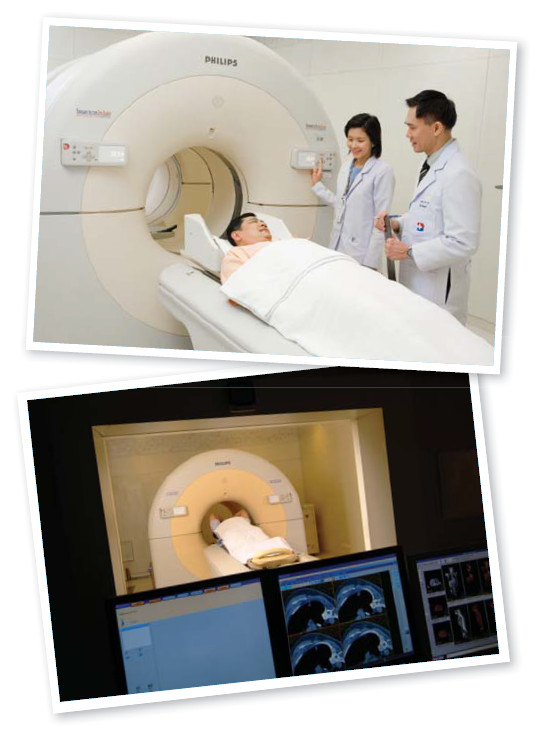
Dr. Samart Rajchadara has worked very hard to educate medical staff and members of the general public about PET/CT. It took many years to source physicians familiar with PET/CT who knew how to use PET/CT properly. The marketing team has arranged several seminars in hospitals and public arenas for Dr. Samart to talk about PET/CT and to present the clinical uses of PET/CT.
With a great contribution from the team who has put in an enormous effort to make the PET service available for the first time in Thailand, we are very proud to have been able to work around the difficulties and problems until the PET service was successfully established for the use of patients. We have faced many problems during the learning and development phases of this project. The team includes physicians, physicists, technicians, nurses, engineers, chemists, and management staff. They are working to continuously improve the quality of services and the quality of processes to keep up with international standards. We have been able to develop the necessary skills and acquire important knowledge about PET/CT and cyclotron-produced radiopharmaceuticals and we are willing to share our valuable knowledge with a wide group of professionals in medical circles.
Acknowledgements